Oxidation of Intracellular and Extracellular Fatty Acids in Skeletal Muscle: Application of kinetic modeling, stable isotopes and liquid chromatography/electrospray ionization ion-trap tandem mass spectrometry technology
- PMID: 23616729
- PMCID: PMC3632343
- DOI: 10.1002/ejlt.200600267
Oxidation of Intracellular and Extracellular Fatty Acids in Skeletal Muscle: Application of kinetic modeling, stable isotopes and liquid chromatography/electrospray ionization ion-trap tandem mass spectrometry technology
Abstract
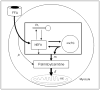
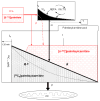
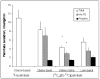
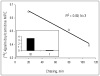
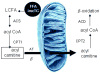
Fatty acids are a major fuel for many tissues and abnormal utilization is implicated in diseases. However, tissue fatty acid oxidation has not been determined reliably in vivo. Furthermore, fatty acid oxidation has not been partitioned into intracellular and extracellular components. In this report, a one-pool model is described that enables direct quantitation of fluxes of intracellular and plasma fatty acids to mitochondria in skeletal muscle using dual stable isotopes and liquid chromatography/electrospray ionization ion-trap tandem mass spectrometry (LC/ESI-itMS2) technology. It is validated by the determination of palmitate oxidation by skeletal muscle in lean and obese rats and the regulation by insulin. Resting postabsorptive intramyocellular and plasma palmitate oxidation by gastrocnemius muscle was determined to be 3.47±0.8 and 2.06±0.5 nmol/g min in lean and 6.96±1.8 and 1.34±0.2 nmol/g min in obese rats, respectively. In obese rats, hyperinsulinemia (1 nmol/l) suppressed intramyocellular (by 59±5% to 2.88±0.3 nmol/g min P<0.05) but not plasma (1.41±0.14 nmol/g min, P>0.05) palmitate oxidation. The fractional turnover rate of palmitoylcarnitine (0.34±0.1/min vs. 0.83±0.2/min, P<0.05) was also suppressed by insulin. In obese and lean rats, there are 83% and 51%, respectively (P=0.08), of plasma fatty acids traverse triglyceride pool before being oxidized. The results demonstrated that the methodology is feasible and sensitive to metabolic alterations and thus can be used to study fatty acid utilization at tissue level in a compartmentalized manner for the firs time.
Keywords: LC-MS; beta-oxidation; compartmentalization; extracellular; intracellular; skeletal muscle; stable isotope.
Figures
Similar articles
-
Muscle type-dependent responses to insulin in intramyocellular triglyceride turnover in obese rats.Obes Res. 2005 Dec;13(12):2081-7. doi: 10.1038/oby.2005.258. Obes Res. 2005. PMID: 16421341
-
High-precision isotopic analysis of palmitoylcarnitine by liquid chromatography/electrospray ionization ion-trap tandem mass spectrometry.Rapid Commun Mass Spectrom. 2006;20(22):3361-6. doi: 10.1002/rcm.2753. Rapid Commun Mass Spectrom. 2006. PMID: 17044121
-
Hyperinsulinemia and skeletal muscle fatty acid trafficking.Am J Physiol Endocrinol Metab. 2013 Aug 15;305(4):E540-8. doi: 10.1152/ajpendo.00143.2013. Epub 2013 Jul 2. Am J Physiol Endocrinol Metab. 2013. PMID: 23820622 Free PMC article.
-
Increased fatty acid uptake and altered fatty acid metabolism in insulin-resistant muscle of obese Zucker rats.Diabetes. 2001 Jun;50(6):1389-96. doi: 10.2337/diabetes.50.6.1389. Diabetes. 2001. PMID: 11375340
-
Forced degradation and impurity profiling: recent trends in analytical perspectives.J Pharm Biomed Anal. 2013 Dec;86:11-35. doi: 10.1016/j.jpba.2013.07.013. Epub 2013 Jul 31. J Pharm Biomed Anal. 2013. PMID: 23969330 Review.
Cited by
-
Unlocking Hydrogenation for C-C Bond Formation: A Brief Overview of Enantioselective Methods.Org Process Res Dev. 2011 Nov 18;15(6):1236-1242. doi: 10.1021/op200195m. Org Process Res Dev. 2011. PMID: 22125398 Free PMC article.
-
Direct, redox-neutral prenylation and geranylation of secondary carbinol C-H bonds: C4-regioselectivity in ruthenium-catalyzed C-C couplings of dienes to α-hydroxy esters.J Am Chem Soc. 2012 Sep 26;134(38):15700-3. doi: 10.1021/ja3075049. Epub 2012 Sep 17. J Am Chem Soc. 2012. PMID: 22985393 Free PMC article.
References
-
- Raatz SK, Bibus D, Thomas W, Kris-Etherton P. Total fat intake modifies plasma fatty acid composition in humans. J Nutr. 2001;131:231–234. - PubMed
-
- Elks ML. Fat oxidation and diabetes of obesity: the Randle hypothesis revisited. Med Hypotheses. 1990;33:257–260. - PubMed
-
- Carley AN, Severson DL. Fatty acid metabolism is enhanced in type 2 diabetic hearts. Biochim Biophys Acta. 2005;1734:112–126. - PubMed
-
- Ferre P. The biology of peroxisome proliferator-activated receptors: relationship with lipid metabolism and insulin sensitivity. Diabetes. 2004;53(Suppl 1):S43–S50. - PubMed
-
- Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, Cooksey RC, Litwin SE, Abel ED. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146:5341–5349. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
