Origin and differentiation of microglia
- PMID: 23616747
- PMCID: PMC3627983
- DOI: 10.3389/fncel.2013.00045
Origin and differentiation of microglia
Abstract
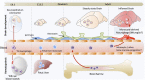
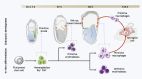
Microglia are the resident macrophage population of the central nervous system (CNS). Adequate microglial function is crucial for a healthy CNS. Microglia are not only the first immune sentinels of infection, contributing to both innate and adaptive immune responses locally, but are also involved in the maintenance of brain homeostasis. Emerging data are showing new and fundamental roles for microglia in the control of neuronal proliferation and differentiation, as well as in the formation of synaptic connections. While microglia have been studied for decades, a long history of experimental misinterpretation meant that their true origins remained debated. However, recent studies on microglial origin indicate that these cells in fact arise early during development from progenitors in the embryonic yolk sac (YS) that seed the brain rudiment and, remarkably, appear to persist there into adulthood. Here, we review the history of microglial cells and discuss the latest advances in our understanding of their origin, differentiation, and homeostasis, which provides new insights into their roles in health and disease.
Keywords: central nervous system; macrophage; microglia; origin; yolk sac.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

