Role of orexin in modulating arousal, feeding, and motivation
- PMID: 23616752
- PMCID: PMC3629303
- DOI: 10.3389/fnbeh.2013.00028
Role of orexin in modulating arousal, feeding, and motivation
Abstract
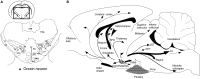
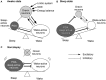
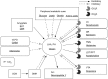
Orexin deficiency results in narcolepsy in humans, dogs, and rodents, suggesting that the orexin system is particularly important for maintenance of wakefulness. However, orexin neurons are "multi-tasking" neurons that regulate sleep/wake states as well as feeding behavior, emotion, and reward processes. Orexin deficiency causes abnormalities in energy homeostasis, stress-related behavior, and reward systems. Orexin excites waking-active monoaminergic and cholinergic neurons in the hypothalamus and brain stem regions to maintain a long, consolidated waking period. Orexin neurons also have reciprocal links with the hypothalamic nuclei, which regulates feeding. Moreover, the responsiveness of orexin neurons to peripheral metabolic cues suggests that these neurons have an important role as a link between energy homeostasis and vigilance states. The link between orexin and the ventral tegmental nucleus serves to motivate an animal to engage in goal-directed behavior. This review focuses on the interaction of orexin neurons with emotion, reward, and energy homeostasis systems. These connectivities are likely to be highly important to maintain proper vigilance states.
Keywords: feeding behavior; hypothalamus; orexin A; orexin receptors; orexins; reward; sleep.
Figures
Similar articles
-
Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system.Pharmacol Rev. 2009 Jun;61(2):162-76. doi: 10.1124/pr.109.001321. Pharmacol Rev. 2009. PMID: 19549926 Review.
-
Orexin neuronal circuitry: role in the regulation of sleep and wakefulness.Front Neuroendocrinol. 2008 Jan;29(1):70-87. doi: 10.1016/j.yfrne.2007.08.001. Epub 2007 Aug 29. Front Neuroendocrinol. 2008. PMID: 17910982 Review.
-
The physiological role of orexin/hypocretin neurons in the regulation of sleep/wakefulness and neuroendocrine functions.Front Endocrinol (Lausanne). 2013 Mar 6;4:18. doi: 10.3389/fendo.2013.00018. eCollection 2013. Front Endocrinol (Lausanne). 2013. PMID: 23508038 Free PMC article.
-
Roles of orexins and orexin receptors in central regulation of feeding behavior and energy homeostasis.CNS Neurol Disord Drug Targets. 2006 Jun;5(3):313-25. doi: 10.2174/187152706777452218. CNS Neurol Disord Drug Targets. 2006. PMID: 16787231 Review.
-
Orexin: a link between energy homeostasis and adaptive behaviour.Curr Opin Clin Nutr Metab Care. 2003 Jul;6(4):353-60. doi: 10.1097/01.mco.0000078995.96795.91. Curr Opin Clin Nutr Metab Care. 2003. PMID: 12806206 Review.
Cited by
-
Low-intensity focused ultrasound attenuates early traumatic brain injury by OX-A/NF-κB/NLRP3 signaling pathway.Aging (Albany NY). 2022 Sep 16;14(18):7455-7469. doi: 10.18632/aging.204290. Epub 2022 Sep 16. Aging (Albany NY). 2022. PMID: 36126193 Free PMC article.
-
Association between severe headache or migraine and lipid accumulation product and visceral adiposity index in adults: a cross-sectional study from NHANES.Lipids Health Dis. 2024 Sep 27;23(1):307. doi: 10.1186/s12944-024-02303-w. Lipids Health Dis. 2024. PMID: 39334367 Free PMC article.
-
Disorders of Body Weight, Sleep and Circadian Rhythm as Manifestations of Hypothalamic Dysfunction in Alzheimer's Disease.Front Cell Neurosci. 2018 Dec 5;12:471. doi: 10.3389/fncel.2018.00471. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30568576 Free PMC article. Review.
-
The circadian clock in the mouse habenula is set by catecholamines.Cell Tissue Res. 2022 Feb;387(2):261-274. doi: 10.1007/s00441-021-03557-x. Epub 2021 Nov 24. Cell Tissue Res. 2022. PMID: 34816282
-
Auditory fear memory retrieval requires BLA-LS and LS-VMH circuitries via GABAergic and dopaminergic neurons.EMBO Rep. 2025 Apr;26(7):1816-1834. doi: 10.1038/s44319-025-00403-x. Epub 2025 Mar 7. EMBO Rep. 2025. PMID: 40055468 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

