GABA and Glutamate Uptake and Metabolism in Retinal Glial (Müller) Cells
- PMID: 23616782
- PMCID: PMC3627989
- DOI: 10.3389/fendo.2013.00048
GABA and Glutamate Uptake and Metabolism in Retinal Glial (Müller) Cells
Abstract
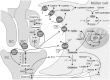
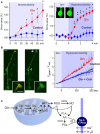
Müller cells, the principal glial cells of the retina, support the synaptic activity by the uptake and metabolization of extracellular neurotransmitters. Müller cells express uptake and exchange systems for various neurotransmitters including glutamate and γ-aminobutyric acid (GABA). Müller cells remove the bulk of extracellular glutamate in the inner retina and contribute to the glutamate clearance around photoreceptor terminals. By the uptake of glutamate, Müller cells are involved in the shaping and termination of the synaptic activity, particularly in the inner retina. Reactive Müller cells are neuroprotective, e.g., by the clearance of excess extracellular glutamate, but may also contribute to neuronal degeneration by a malfunctioning or even reversal of glial glutamate transporters, or by a downregulation of the key enzyme, glutamine synthetase. This review summarizes the present knowledge about the role of Müller cells in the clearance and metabolization of extracellular glutamate and GABA. Some major pathways of GABA and glutamate metabolism in Müller cells are described; these pathways are involved in the glutamate-glutamine cycle of the retina, in the defense against oxidative stress via the production of glutathione, and in the production of substrates for the neuronal energy metabolism.
Keywords: GABA; Müller cells; glutamate; recycling; retina; retinal pathology.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

