HIS-Based Support of Follow-Up Documentation - Concept and Implementation for Clinical Studies
- PMID: 23616857
- PMCID: PMC3631912
- DOI: 10.4338/ACI-2010-08-RA-0047
HIS-Based Support of Follow-Up Documentation - Concept and Implementation for Clinical Studies
Abstract
Objective: Follow-up data must be collected according to the protocol of each clinical study, i.e. at certain time points. Missing follow-up information is a critical problem and may impede or bias the analysis of study data and result in delays. Moreover, additional patient recruitment may be necessary due to incomplete follow-up data. Current electronic data capture (EDC) systems in clinical studies are usually separated from hospital information systems (HIS) and therefore can provide limited functionality to support clinical workflow. In two case studies, we assessed the feasibility of HIS-based support of follow-up documentation.
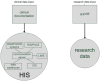
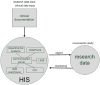
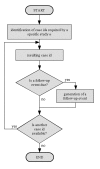
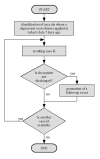
Methods: We have developed a data model and a HIS-based workflow to provide follow-up forms according to clinical study protocols. If a follow-up form was due, a database procedure created a follow-up event which was translated by a communication server into an HL7 message and transferred to the import interface of the clinical information system (CIS). This procedure generated the required follow-up form and enqueued a link to it in a work list of the relating study nurses and study physicians, respectively.
Results: A HIS-based follow-up system automatically generated follow-up forms as defined by a clinical study protocol. These forms were scheduled into work lists of study nurses and study physicians. This system was integrated into the clinical workflow of two clinical studies. In a study from nuclear medicine, each scenario from the test concept according to the protocol of the single photon emission computer tomography/computer tomography (SPECT/CT) study was simulated and each scenario passed the test. For a study in psychiatry, 128 follow-up forms were automatically generated within 27 weeks, on average five forms per week (maximum 12, minimum 1 form per week).
Conclusion: HIS-based support of follow-up documentation in clinical studies is technically feasible and can support compliance with study protocols.
Keywords: Follow-up; clinical studies; completeness; hospital information system; single source information system.
Figures






Similar articles
-
HIS-based Kaplan-Meier plots--a single source approach for documenting and reusing routine survival information.BMC Med Inform Decis Mak. 2011 Feb 16;11:11. doi: 10.1186/1472-6947-11-11. BMC Med Inform Decis Mak. 2011. PMID: 21324182 Free PMC article.
-
Concept and implementation of a computer-based reminder system to increase completeness in clinical documentation.Int J Med Inform. 2011 May;80(5):351-8. doi: 10.1016/j.ijmedinf.2011.02.004. Epub 2011 Mar 15. Int J Med Inform. 2011. PMID: 21411365
-
Concept and Implementation of a Single Source Information System in Nuclear Medicine for Myocardial Scintigraphy (SPECT-CT data).Appl Clin Inform. 2010 Mar 31;1(1):50-67. doi: 10.4338/ACI-2009-12-RA-0017. Print 2010. Appl Clin Inform. 2010. PMID: 23616827 Free PMC article.
-
[Cost-effectiveness analysis of schizophrenic patient care settings: impact of an atypical antipsychotic under long-acting injection formulation].Encephale. 2005 Mar-Apr;31(2):235-46. doi: 10.1016/s0013-7006(05)82390-5. Encephale. 2005. PMID: 15959450 Review. French.
-
Real-Time Electronic Dashboard Technology and Its Use to Improve Pediatric Radiology Workflow.Curr Probl Diagn Radiol. 2018 Jan-Feb;47(1):3-5. doi: 10.1067/j.cpradiol.2017.03.002. Epub 2017 Mar 19. Curr Probl Diagn Radiol. 2018. PMID: 28533102 Review.
Cited by
-
CIS-based registration of quality of life in a single source approach.BMC Med Inform Decis Mak. 2011 Apr 21;11:26. doi: 10.1186/1472-6947-11-26. BMC Med Inform Decis Mak. 2011. PMID: 21510866 Free PMC article.
References
-
- Chan KS, Fowles J, Weiner JP. Electronic health records and reliability and validity of quality measures. A review of the literature. Medical Care Research and Review. Forthcoming 2010. doi:10.1177/1077558709359007 PMid:20150441 - PubMed
-
- Ammenwerth E, HP Spötl. The time needed for clinical documentation versus direct patient care. Methods Inf Med 2009; 48: 84-91 PMid:19151888 - PubMed
-
- Forster M, Bailey C, Brinkhof MW, Graber C, Boulle A, Spohr M, et al. Electronic medical record systems, data quality and loss to follow-up: survey of antiretroviral therapy programmes in resource-limited settings. Bulletin of the World Health Organization 2008; 86: 939-947 doi:10.2471/BLT.07.049908 PMid:19142294 PMCid:2649575 - PMC - PubMed
-
- Dugas M, Breil B, Thiemann V, Lechtenbörger J, Vossen G. Single source information system to connect patient care and clinical research. Stud Health Technol Inform 2009; 150: 61-65 PMid:19745267 - PubMed
LinkOut - more resources
Full Text Sources

