Basal aurora kinase B activity is sufficient for histone H3 phosphorylation in prophase
- PMID: 23616922
- PMCID: PMC3625866
- DOI: 10.1242/bio.20133079
Basal aurora kinase B activity is sufficient for histone H3 phosphorylation in prophase
Abstract
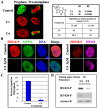
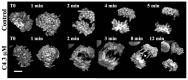
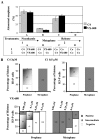
Histone H3 phosphorylation is the hallmark of mitosis deposited by aurora kinase B. Benzo[e]pyridoindoles are a family of potent, broad, ATP-competitive aurora kinase inhibitors. However, benzo[e]pyridoindole C4 only inhibits histone H3 phosphorylation in prophase but not in metaphase. Under the C4 treatment, the cells enter into mitosis with dephosphorylated histone H3, assemble chromosomes normally and progress to metaphase, and then to anaphase. C4 also induces lagging chromosome in anaphase but we demonstrated that these chromosome compaction defects are not related to the absence of H3 phosphorylation in prophase. As a result of C4 action, mitosis lasts longer and the cell cycle is slowed down. We reproduced the mitotic defects with reduced concentrations of potent pan aurora kinase as well as with a specific aurora B ATP-competitive inhibitor; we therefore propose that histone H3 phosphorylation and anaphase chromosome compaction involve the basal activity of aurora kinase B. Our data suggest that aurora kinase B is progressively activated at mitosis entry and at anaphase onset. The full activation of aurora kinase B by its partners, in prometaphase, induces a shift in the catalytic domain of aurora B that modifies its affinity for ATP. These waves of activation/deactivation of aurora B correspond to different conformations of the chromosomal complex revealed by FRAP. The presence of lagging chromosomes may have deleterious consequences on the daughter cells and, unfortunately, the situation may be encountered in patients receiving treatment with aurora kinase inhibitors.
Keywords: Aurora kinase; Cancer; Chromosomal passenger complex; Chromosome compaction; Histone H3 phosphorylation; Kinase inhibitors; Survivin.
Conflict of interest statement
Figures





Similar articles
-
Chromosome segregation regulation in human zygotes: altered mitotic histone phosphorylation dynamics underlying centromeric targeting of the chromosomal passenger complex.Hum Reprod. 2015 Oct;30(10):2275-91. doi: 10.1093/humrep/dev186. Epub 2015 Jul 29. Hum Reprod. 2015. PMID: 26223676
-
Aurora-B phosphorylates Histone H3 at serine28 with regard to the mitotic chromosome condensation.Genes Cells. 2002 Jan;7(1):11-7. doi: 10.1046/j.1356-9597.2001.00498.x. Genes Cells. 2002. PMID: 11856369
-
Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases.Mol Cell Biol. 2002 Feb;22(3):874-85. doi: 10.1128/MCB.22.3.874-885.2002. Mol Cell Biol. 2002. PMID: 11784863 Free PMC article.
-
Role of chromosomal passenger complex in chromosome segregation and cytokinesis.Cell Struct Funct. 2001 Dec;26(6):653-7. doi: 10.1247/csf.26.653. Cell Struct Funct. 2001. PMID: 11942622 Review.
-
Chromosomal passengers: the four-dimensional regulation of mitotic events.Chromosoma. 2004 Nov;113(5):211-22. doi: 10.1007/s00412-004-0307-3. Epub 2004 Sep 4. Chromosoma. 2004. PMID: 15351889 Review.
Cited by
-
Screening of compounds to identify novel epigenetic regulatory factors that affect innate immune memory in macrophages.Sci Rep. 2022 Feb 3;12(1):1912. doi: 10.1038/s41598-022-05929-x. Sci Rep. 2022. PMID: 35115604 Free PMC article.
-
Discovery of benzo[e]pyridoindolones as kinase inhibitors that disrupt mitosis exit while erasing AMPK-Thr172 phosphorylation on the spindle.Oncotarget. 2015 Sep 8;6(26):22152-66. doi: 10.18632/oncotarget.4158. Oncotarget. 2015. PMID: 26247630 Free PMC article.
-
Deciphering the potential role of post-translational modifications of histones in gastrointestinal cancers: a proteomics-based review with therapeutic challenges and opportunities.Front Oncol. 2024 Oct 21;14:1481426. doi: 10.3389/fonc.2024.1481426. eCollection 2024. Front Oncol. 2024. PMID: 39497715 Free PMC article. Review.
-
The Marine Natural Compound Dragmacidin D Selectively Induces Apoptosis in Triple-Negative Breast Cancer Spheroids.Mar Drugs. 2023 Dec 15;21(12):642. doi: 10.3390/md21120642. Mar Drugs. 2023. PMID: 38132962 Free PMC article.
-
Elongation during segmentation shows axial variability, low mitotic rates, and synchronized cell cycle domains in the crustacean, Thamnocephalus platyurus.Evodevo. 2020 Jan 18;11:1. doi: 10.1186/s13227-020-0147-0. eCollection 2020. Evodevo. 2020. PMID: 31988708 Free PMC article.
References
-
- Adams R. R., Maiato H., Earnshaw W. C., Carmena M. (2001). Essential roles of Drosophila inner centromere protein (INCENP) and Aurora B in histone H3 phosphorylation, metaphase chromosome alignment, kinetochore disjunction, and chromosome segregation. J. Cell Biol. 153, 865–880 10.1083/jcb.153.4.865 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

