Moderate excess of pyruvate augments osteoclastogenesis
- PMID: 23616923
- PMCID: PMC3625867
- DOI: 10.1242/bio.20133269
Moderate excess of pyruvate augments osteoclastogenesis
Abstract
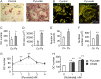
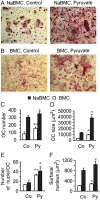
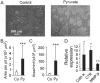
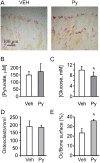
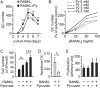
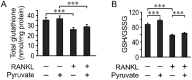
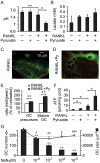
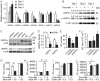
Cell differentiation leads to adaptive changes in energy metabolism. Conversely, hyperglycemia induces malfunction of many body systems, including bone, suggesting that energy metabolism reciprocally affects cell differentiation. We investigated how the differentiation of bone-resorbing osteoclasts, large polykaryons formed through fusion and growth of cells of monocytic origin, is affected by excess of energy substrate pyruvate and how energy metabolism changes during osteoclast differentiation. Surprisingly, small increases in pyruvate (1-2 mM above basal levels) augmented osteoclastogenesis in vitro and in vivo, while larger increases were not effective in vitro. Osteoclast differentiation increased cell mitochondrial activity and ATP levels, which were further augmented in energy-rich conditions. Conversely, the inhibition of respiration significantly reduced osteoclast number and size. AMP-activated protein kinase (AMPK) acts as a metabolic sensor, which is inhibited in energy-rich conditions. We found that osteoclast differentiation was associated with an increase in AMPK levels and a change in AMPK isoform composition. Increased osteoclast size induced by pyruvate (1 mM above basal levels) was prevented in the presence of AMPK activator 5-amino-4-imidazole carboxamide ribonucleotide (AICAR). In keeping, inhibition of AMPK using dorsomorphin or siRNA to AMPKγ increased osteoclast size in control cultures to the level observed in the presence of pyruvate. Thus, we have found that a moderate excess of pyruvate enhances osteoclastogenesis, and that AMPK acts to tailor osteoclastogenesis to a cell's bioenergetics capacity.
Keywords: AMPK; ATP; Hyperglycemia; Osteoclastogenesis; Pyruvate.
Conflict of interest statement
Figures
Similar articles
-
CTRP3 acts as a negative regulator of osteoclastogenesis through AMPK-c-Fos-NFATc1 signaling in vitro and RANKL-induced calvarial bone destruction in vivo.Bone. 2015 Oct;79:242-51. doi: 10.1016/j.bone.2015.06.011. Epub 2015 Jun 21. Bone. 2015. PMID: 26103094
-
Regulation of Osteoclast Growth and Fusion by mTOR/raptor and mTOR/rictor/Akt.Front Cell Dev Biol. 2017 May 18;5:54. doi: 10.3389/fcell.2017.00054. eCollection 2017. Front Cell Dev Biol. 2017. PMID: 28573133 Free PMC article.
-
Osteoclast precursors display dynamic metabolic shifts toward accelerated glucose metabolism at an early stage of RANKL-stimulated osteoclast differentiation.Cell Physiol Biochem. 2007;20(6):935-46. doi: 10.1159/000110454. Cell Physiol Biochem. 2007. PMID: 17982276
-
AMP-activated protein kinase (AMPK) regulates autophagy, inflammation and immunity and contributes to osteoclast differentiation and functionabs.Biol Cell. 2020 Sep;112(9):251-264. doi: 10.1111/boc.202000008. Epub 2020 Jun 8. Biol Cell. 2020. PMID: 32445585 Review.
-
Role of Altered Metabolic Microenvironment in Osteolytic Metastasis.Front Cell Dev Biol. 2020 Jun 5;8:435. doi: 10.3389/fcell.2020.00435. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32582711 Free PMC article. Review.
Cited by
-
The Macrophage-Osteoclast Axis in Osteoimmunity and Osteo-Related Diseases.Front Immunol. 2021 Mar 31;12:664871. doi: 10.3389/fimmu.2021.664871. eCollection 2021. Front Immunol. 2021. PMID: 33868316 Free PMC article. Review.
-
Fructose 1,6-bisphosphate inhibits osteoclastogenesis by attenuating RANKL-induced NF-κB/NFATc-1.Inflamm Res. 2019 May;68(5):415-421. doi: 10.1007/s00011-019-01228-w. Epub 2019 Mar 29. Inflamm Res. 2019. PMID: 30927049
-
Wnt7b Inhibits Osteoclastogenesis via AKT Activation and Glucose Metabolic Rewiring.Front Cell Dev Biol. 2021 Nov 22;9:771336. doi: 10.3389/fcell.2021.771336. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34881243 Free PMC article.
-
The investigation of energy metabolism in osteoblasts and osteoclasts.Hua Xi Kou Qiang Yi Xue Za Zhi. 2021 Oct 1;39(5):501-509. doi: 10.7518/hxkq.2021.05.002. Hua Xi Kou Qiang Yi Xue Za Zhi. 2021. PMID: 34636196 Free PMC article. Chinese, English.
-
Energy Metabolism of the Osteoblast: Implications for Osteoporosis.Endocr Rev. 2017 Jun 1;38(3):255-266. doi: 10.1210/er.2017-00064. Endocr Rev. 2017. PMID: 28472361 Free PMC article. Review.
References
-
- Barnerias C., Saudubray J. M., Touati G., De Lonlay P., Dulac O., Ponsot G., Marsac C., Brivet M., Desguerre I. (2010). Pyruvate dehydrogenase complex deficiency: four neurological phenotypes with differing pathogenesis. Dev. Med. Child Neurol. 52, e1–e9 10.1111/j.1469-8749.2009.03541.x - DOI - PubMed
-
- Bassenge E., Sommer O., Schwemmer M., Bünger R. (2000). Antioxidant pyruvate inhibits cardiac formation of reactive oxygen species through changes in redox state. Am. J. Physiol. 279, H2431–H2438. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

