Overview of methods for comparing the efficacies of drugs in the absence of head-to-head clinical trial data
- PMID: 23617453
- PMCID: PMC3895352
- DOI: 10.1111/bcp.12150
Overview of methods for comparing the efficacies of drugs in the absence of head-to-head clinical trial data
Abstract
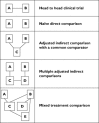
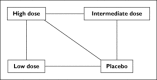
In most therapeutic areas, multiple drug options are increasingly becoming available, but there is often a lack of evidence from head-to-head clinical trials that allows for direct comparison of the efficacy and/or safety of one drug vs. another. This review provides an introduction to, and overview of, common methods used for comparing drugs in the absence of head-to-head clinical trial evidence. Naïve direct comparisons are in most instances inappropriate and should only be used for exploratory purposes and when no other options are possible. Adjusted indirect comparisons are currently the most commonly accepted method and use links through one or more common comparators. Mixed treatment comparisons (MTCs) use Bayesian statistical models to incorporate all available data for a drug, even data that are not relevant to the comparator drug. MTCs reduce uncertainty but have not yet been widely accepted by researchers, nor drug regulatory and reimbursement authorities. All indirect analyses are based on the same underlying assumption as meta-analyses, namely that the study populations in the trials being compared are similar.
Keywords: adjusted indirect comparison; mixed treatment comparison; naïve direct comparison.
© 2013 The British Pharmacological Society.
Figures
References
-
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–691. - PubMed
-
- PBAC. Canberra, Australia: 2008. Australian Department of Health and Ageing: Guidelines for preparing submissions to the Pharmaceutical Benefits Advisory Committee (PBAC), Australian Department of Health and Ageing, Editor.
-
- NICE. Guide to the methods of technology appraisal, N.I.f.H.a.C. Excellence, Editor 2008.
-
- Well GA, Sultan SA, Chen L, Khan M, Coyle D. Indirect Evidence: Indirect Treatment Comparisons in meta-Analysis, C.A.f.D.a.T.i. Health, Editor 2009.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

