Development of a transmission model for dengue virus
- PMID: 23617898
- PMCID: PMC3659020
- DOI: 10.1186/1743-422X-10-127
Development of a transmission model for dengue virus
Abstract
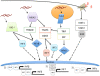
Background: Dengue virus (DENV) research has historically been hampered by the lack of a susceptible vertebrate transmission model. Recently, there has been progress towards such models using several varieties of knockout mice, particularly those deficient in type I and II interferon receptors. Based on the critical nature of the type I interferon response in limiting DENV infection establishment, we assessed the permissiveness of a mouse strain with a blunted type I interferon response via gene deficiencies in interferon regulatory factors 3 and 7 (IRF3/7 -/- -/-) with regards to DENV transmission success. We investigated the possibility of transmission to the mouse by needle and infectious mosquito, and subsequent transmission back to mosquito from an infected animal during its viremic period.
Methods: Mice were inoculated subcutaneously with non-mouse adapted DENV-2 strain 1232 and serum was tested for viral load and cytokine production each day. Additionally, mosquitoes were orally challenged with the same DENV-2 strain via artificial membrane feeder, and then allowed to forage or naïve mice. Subsequently, we determined acquisition potential by allowing naïve mosquitoes on forage on exposed mice during their viremic period.
Results: Both needle inoculation and infectious mosquito bite(s) resulted in 100% infection. Significant differences between these groups in viremia on the two days leading to peak viremia were observed, though no significant difference in cytokine production was seen. Through our determination of transmission and acquisition potentials, the transmission cycle (mouse-to mosquito-to mouse) was completed. We confirmed that the IRF3/7 -/- -/- mouse supports DENV replication and is competent for transmission experiments, with the ability to use a non-mouse adapted DENV-2 strain. A significant finding of this study was that this IRF3/7 -/- -/- mouse strain was able to be infected by and transmit virus to mosquitoes, thus providing means to replicate the natural transmission cycle of DENV.
Conclusion: As there is currently no approved vaccine for DENV, public health monitoring and a greater understanding of transmission dynamics leading to outbreak events are critical. The further characterization of DENV using this model will expand knowledge of key entomological, virological and immunological components of infection establishment and transmission events.
Figures




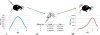
References
-
- WHO. Fact Sheet No. 117: Dengue and severe dengue. 2012. [cited December 8, 2012]; Available from: http://www.who.int/mediacentre/factsheets/fs117/en/
-
- Teixeira MG, Costa Mda C, Barreto F, Barreto ML. Dengue: twenty-five years since reemergence in Brazil. Cad Saude Publica. 2009;25(Suppl 1):S7–S18. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

