Organometallic Iridium(III) anticancer complexes with new mechanisms of action: NCI-60 screening, mitochondrial targeting, and apoptosis
- PMID: 23618382
- PMCID: PMC3691721
- DOI: 10.1021/cb400070a
Organometallic Iridium(III) anticancer complexes with new mechanisms of action: NCI-60 screening, mitochondrial targeting, and apoptosis
Abstract
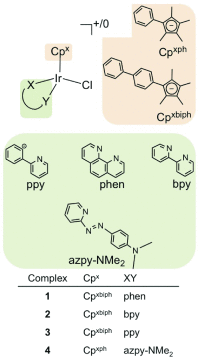
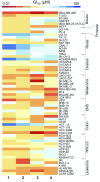
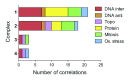
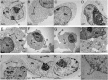
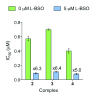
Platinum complexes related to cisplatin, cis-[PtCl2(NH3)2], are successful anticancer drugs; however, other transition metal complexes offer potential for combating cisplatin resistance, decreasing side effects, and widening the spectrum of activity. Organometallic half-sandwich iridium (Ir(III)) complexes [Ir(Cp(x))(XY)Cl](+/0) (Cp(x) = biphenyltetramethylcyclopentadienyl and XY = phenanthroline (1), bipyridine (2), or phenylpyridine (3)) all hydrolyze rapidly, forming monofunctional G adducts on DNA with additional intercalation of the phenyl substituents on the Cp(x) ring. In comparison, highly potent complex 4 (Cp(x) = phenyltetramethylcyclopentadienyl and XY = N,N-dimethylphenylazopyridine) does not hydrolyze. All show higher potency toward A2780 human ovarian cancer cells compared to cisplatin, with 1, 3, and 4 also demonstrating higher potency in the National Cancer Institute (NCI) NCI-60 cell-line screen. Use of the NCI COMPARE algorithm (which predicts mechanisms of action (MoAs) for emerging anticancer compounds by correlating NCI-60 patterns of sensitivity) shows that the MoA of these Ir(III) complexes has no correlation to cisplatin (or oxaliplatin), with 3 and 4 emerging as particularly novel compounds. Those findings by COMPARE were experimentally probed by transmission electron microscopy (TEM) of A2780 cells exposed to 1, showing mitochondrial swelling and activation of apoptosis after 24 h. Significant changes in mitochondrial membrane polarization were detected by flow cytometry, and the potency of the complexes was enhanced ca. 5× by co-administration with a low concentration (5 μM) of the γ-glutamyl cysteine synthetase inhibitor L-buthionine sulfoximine (L-BSO). These studies reveal potential polypharmacology of organometallic Ir(III) complexes, with MoA and cell selectivity governed by structural changes in the chelating ligands.
Figures

Similar articles
-
Novel and Versatile Imine-N-Heterocyclic Carbene Half-Sandwich Iridium(III) Complexes as Lysosome-Targeted Anticancer Agents.Inorg Chem. 2018 Sep 4;57(17):11087-11098. doi: 10.1021/acs.inorgchem.8b01656. Epub 2018 Aug 22. Inorg Chem. 2018. PMID: 30133276
-
Potential anticancer agent for selective damage to mitochondria or lysosomes: Naphthalimide-modified fluorescent biomarker half-sandwich iridium (III) and ruthenium (II) complexes.Eur J Med Chem. 2019 Nov 1;181:111599. doi: 10.1016/j.ejmech.2019.111599. Epub 2019 Aug 6. Eur J Med Chem. 2019. PMID: 31408807
-
Half-Sandwich Iridium(III) and Ruthenium(II) Complexes Containing P^P-Chelating Ligands: A New Class of Potent Anticancer Agents with Unusual Redox Features.Inorg Chem. 2018 Feb 19;57(4):1705-1716. doi: 10.1021/acs.inorgchem.7b01959. Epub 2018 Feb 5. Inorg Chem. 2018. PMID: 29400963
-
Organoiridium complexes: anticancer agents and catalysts.Acc Chem Res. 2014 Apr 15;47(4):1174-85. doi: 10.1021/ar400266c. Epub 2014 Feb 20. Acc Chem Res. 2014. PMID: 24555658 Free PMC article. Review.
-
Iridium(III) Complexes Targeting Apoptotic Cell Death in Cancer Cells.Molecules. 2019 Jul 28;24(15):2739. doi: 10.3390/molecules24152739. Molecules. 2019. PMID: 31357712 Free PMC article. Review.
Cited by
-
Metal-Based Anticancer Complexes and p53: How Much Do We Know?Cancers (Basel). 2023 May 19;15(10):2834. doi: 10.3390/cancers15102834. Cancers (Basel). 2023. PMID: 37345171 Free PMC article. Review.
-
Novel ethanocycloheptono [3,4,5-kl]benzo[a]xanthene induces apoptosis in BEL-7402 cells.Mol Cell Biochem. 2018 Aug;445(1-2):145-156. doi: 10.1007/s11010-017-3260-1. Epub 2018 Jan 27. Mol Cell Biochem. 2018. PMID: 29380241
-
Synthesis and Biological Evaluation of Novel 1H-Benzo[d]imidazole Derivatives as Potential Anticancer Agents Targeting Human Topoisomerase I.ACS Omega. 2022 Jan 10;7(3):2861-2880. doi: 10.1021/acsomega.1c05743. eCollection 2022 Jan 25. ACS Omega. 2022. PMID: 35097282 Free PMC article.
-
Five-Membered Ring Peroxide Selectively Initiates Ferroptosis in Cancer Cells.ACS Chem Biol. 2016 May 20;11(5):1305-12. doi: 10.1021/acschembio.5b00900. Epub 2016 Mar 1. ACS Chem Biol. 2016. PMID: 26797166 Free PMC article.
-
Synthesis, Molecular Structure and Cytotoxicity of Molecular Materials Based on Water Soluble Half-Sandwich Rh(III) and Ir(III) Tetranuclear Metalla-Cycles.Materials (Basel). 2013 Nov 20;6(11):5352-5366. doi: 10.3390/ma6115352. Materials (Basel). 2013. PMID: 28788394 Free PMC article.
References
-
- Monneret C. (2011) Platinum anticancer drugs. From serendipity to rational design. Ann. Pharm. Fr. 69, 286–295. - PubMed
-
- Kelland L. (2007) The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 7, 573–584. - PubMed
-
- Siddik Z. (2003) Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 22, 7265–7279. - PubMed
-
- Garbutcheon-Singh K. B.; Grant M. P.; Harper B. W.; Krause-Heuer A. M.; Manohar M.; Orkey N.; Aldrich-Wright J. R. (2011) Transition metal based anticancer drugs. Curr. Top. Med. Chem. 11, 521–542. - PubMed
-
- Hannon M. J. (2007) Metal-based anticancer drugs: From a past anchored in platinum chemistry to a post-genomic future of diverse chemistry and biology. Pure Appl. Chem. 79, 2243–2261.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

