Organometallic Iridium(III) anticancer complexes with new mechanisms of action: NCI-60 screening, mitochondrial targeting, and apoptosis
- PMID: 23618382
- PMCID: PMC3691721
- DOI: 10.1021/cb400070a
Organometallic Iridium(III) anticancer complexes with new mechanisms of action: NCI-60 screening, mitochondrial targeting, and apoptosis
Abstract
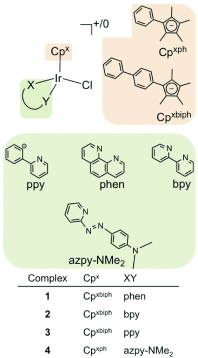
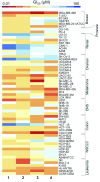
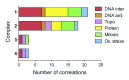
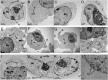
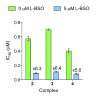
Platinum complexes related to cisplatin, cis-[PtCl2(NH3)2], are successful anticancer drugs; however, other transition metal complexes offer potential for combating cisplatin resistance, decreasing side effects, and widening the spectrum of activity. Organometallic half-sandwich iridium (Ir(III)) complexes [Ir(Cp(x))(XY)Cl](+/0) (Cp(x) = biphenyltetramethylcyclopentadienyl and XY = phenanthroline (1), bipyridine (2), or phenylpyridine (3)) all hydrolyze rapidly, forming monofunctional G adducts on DNA with additional intercalation of the phenyl substituents on the Cp(x) ring. In comparison, highly potent complex 4 (Cp(x) = phenyltetramethylcyclopentadienyl and XY = N,N-dimethylphenylazopyridine) does not hydrolyze. All show higher potency toward A2780 human ovarian cancer cells compared to cisplatin, with 1, 3, and 4 also demonstrating higher potency in the National Cancer Institute (NCI) NCI-60 cell-line screen. Use of the NCI COMPARE algorithm (which predicts mechanisms of action (MoAs) for emerging anticancer compounds by correlating NCI-60 patterns of sensitivity) shows that the MoA of these Ir(III) complexes has no correlation to cisplatin (or oxaliplatin), with 3 and 4 emerging as particularly novel compounds. Those findings by COMPARE were experimentally probed by transmission electron microscopy (TEM) of A2780 cells exposed to 1, showing mitochondrial swelling and activation of apoptosis after 24 h. Significant changes in mitochondrial membrane polarization were detected by flow cytometry, and the potency of the complexes was enhanced ca. 5× by co-administration with a low concentration (5 μM) of the γ-glutamyl cysteine synthetase inhibitor L-buthionine sulfoximine (L-BSO). These studies reveal potential polypharmacology of organometallic Ir(III) complexes, with MoA and cell selectivity governed by structural changes in the chelating ligands.
Figures

References
-
- Monneret C. (2011) Platinum anticancer drugs. From serendipity to rational design. Ann. Pharm. Fr. 69, 286–295. - PubMed
-
- Kelland L. (2007) The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 7, 573–584. - PubMed
-
- Siddik Z. (2003) Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene 22, 7265–7279. - PubMed
-
- Garbutcheon-Singh K. B.; Grant M. P.; Harper B. W.; Krause-Heuer A. M.; Manohar M.; Orkey N.; Aldrich-Wright J. R. (2011) Transition metal based anticancer drugs. Curr. Top. Med. Chem. 11, 521–542. - PubMed
-
- Hannon M. J. (2007) Metal-based anticancer drugs: From a past anchored in platinum chemistry to a post-genomic future of diverse chemistry and biology. Pure Appl. Chem. 79, 2243–2261.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

