Effects of steroid hormones on differentiated glandular epithelial and stromal cells in a three dimensional cell culture model of the canine endometrium
- PMID: 23618385
- PMCID: PMC3660264
- DOI: 10.1186/1746-6148-9-86
Effects of steroid hormones on differentiated glandular epithelial and stromal cells in a three dimensional cell culture model of the canine endometrium
Abstract
Background: Oestrogens and progesterone have a significant impact on the endometrium during the canine oestrous cycle. Their receptors mediate plasma steroid hormone levels and are expressed in several endometrial cell types. Altered steroid receptor expression patterns are involved in serious uterine diseases; however the mechanisms of hormone action during pathogenesis in these tissues remain unclear. The development of 3D culture systems of canine endometrial cells provides an opportunity for the effects of steroid hormones to be quantitatively assessed in a more in vivo-like setting. The present study aimed to determine the effects of the steroid hormones 17β-estradiol (E) and progesterone (P) on the expression of the oestrogen and progesterone receptors (ER and PR), and on proliferative activity, in a 3D co-culture system of canine uterine origin, comprising differentiated endometrial glands, and stromal cells (SCs).
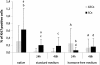
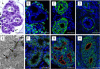
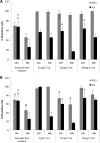
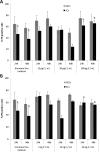
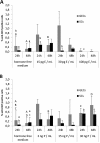
Results: Morphology, differentiation, and apical-basolateral polarity of cultured glandular epithelial cells (GECs) were comparable to those in native uterine tissue as assessed by immunohistochemistry using differentiation markers (β-catenin, laminin), lectin histochemistry, and transmission electron microscopy. Supplementation of our 3D-culture system with E (at 15, 30 and 100 pg/mL) resulted in constant levels of ER expression in GECs, but reduced expression levels in SCs. PR expression was reduced in both GECs and SCs following treatment with E. 3 ng/mL P resulted in increased ER expression in GECs, but a decrease in SCs. PR expression in GECs increased in all P-treated groups, whereas PRs in SCs decreased with the lowest and highest doses, but increased with the middle dose of treatment. Proliferative activity, assessed by Ki67 staining, remained below 1% in all assays and cell types.
Conclusions: The present study demonstrates the applicability of our 3D organotypic canine endometrium-derived culture system for cellular-level studies. 3D cultures represent near-physiological systems allowing reproducible quantitative experimentation, thus reducing the need to experiment on living animals. The results of the present investigation emphasize the importance of co-culture of the uterine glands with SCs, as it was shown that the responsiveness of the different cell types to steroid hormones were divergent in the 3D cell culture model.
Figures


Similar articles
-
Steroid receptors in canine endometrial cells can be regulated by estrogen and progesterone under in vitro conditions.Theriogenology. 2004 Apr 1;61(5):963-76. doi: 10.1016/j.theriogenology.2003.07.002. Theriogenology. 2004. PMID: 14757480
-
Human endometrial epithelial telomerase is important for epithelial proliferation and glandular formation with potential implications in endometriosis.Hum Reprod. 2015 Dec;30(12):2816-28. doi: 10.1093/humrep/dev267. Epub 2015 Oct 25. Hum Reprod. 2015. PMID: 26498179
-
A novel organotypic culture model for normal human endometrium: regulation of epithelial cell proliferation by estradiol and medroxyprogesterone acetate.Hum Reprod. 2005 Apr;20(4):864-71. doi: 10.1093/humrep/deh722. Epub 2005 Jan 21. Hum Reprod. 2005. PMID: 15665014
-
Hormonal regulation and localization of estrogen, progestin and androgen receptors in the endometrium of nonhuman primates: effects of progesterone receptor antagonists.Arch Histol Cytol. 2004 Dec;67(5):393-409. doi: 10.1679/aohc.67.393. Arch Histol Cytol. 2004. PMID: 15781981 Review.
-
Marker molecules of human endometrial differentiation can be hormonally regulated under in-vitro conditions as in-vivo.Hum Reprod Update. 1998 Sep-Oct;4(5):539-49. doi: 10.1093/humupd/4.5.539. Hum Reprod Update. 1998. PMID: 10027607 Review.
Cited by
-
Morphological and Immunohistochemical Characterization of Canine Osteosarcoma Spheroid Cell Cultures.Anat Histol Embryol. 2016 Jun;45(3):219-30. doi: 10.1111/ahe.12190. Epub 2015 Aug 19. Anat Histol Embryol. 2016. PMID: 26287450 Free PMC article.
-
Morphological changes in bitches endometrium affected by cystic endometrial hyperplasia - pyometra complex - the value of histopathological examination.BMC Vet Res. 2021 Apr 26;17(1):174. doi: 10.1186/s12917-021-02875-0. BMC Vet Res. 2021. PMID: 33902588 Free PMC article.
-
Changes in surface morphology, lectin staining, and gene expression of caprine endometrium exposed to estradiol, progesterone, and mifepristone in vitro.Iran J Vet Res. 2022;23(1):61-68. doi: 10.22099/IJVR.2021.41508.6036. Iran J Vet Res. 2022. PMID: 35782354 Free PMC article.
-
Morphological, Ultrastructural, and Molecular Aspects of In Vitro Mouse Embryo Implantation on Human Endometrial Mesenchymal Stromal Cells in The Presence of Steroid Hormones as An Implantation Model.Cell J. 2018 Oct;20(3):369-376. doi: 10.22074/cellj.2018.5221. Epub 2018 May 15. Cell J. 2018. PMID: 29845791 Free PMC article.
-
Androgen Receptors in Human Breast Cancer and Female Canine Mammary Tumors.Molecules. 2025 Mar 22;30(7):1411. doi: 10.3390/molecules30071411. Molecules. 2025. PMID: 40286049 Free PMC article. Review.
References
-
- Noakes DE, Dhaliwal GK, England GC. Cystic endometrial hyperplasia/pyometra in dogs: a review of the causes and pathogenesis. J Reprod Fertil Suppl. 2001;57:395–406. - PubMed
-
- Stadler K, Handler J, Schoenkypl S, Walter I. A three-dimensional culture model of canine uterine glands. In Vitro Cell Dev Biol Anim. 2009;45(1–2):35–43. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

