Characterization of non-hemodynamic functional signal measured by spin-lock fMRI
- PMID: 23618601
- PMCID: PMC3685173
- DOI: 10.1016/j.neuroimage.2013.04.045
Characterization of non-hemodynamic functional signal measured by spin-lock fMRI
Abstract
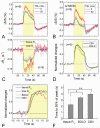
Current functional MRI techniques measure hemodynamic changes induced by neural activity. Alternative measurement of signals originated from tissue is desirable and may be achieved using T1ρ, the spin-lattice relaxation time in the rotating-frame, which is measured by spin-lock MRI. Functional T1ρ changes in the brain can have contributions from vascular dilation, tissue acidosis, and potentially other contributions. When the blood contributions were suppressed with a contrast agent at 9.4 T, a small tissue-originated T1ρ change was consistently observed at the middle cortical layers of cat visual cortex during visual stimulation, which had different dynamic characteristics compared to hemodynamic fMRI such as a faster response and no post-stimulus undershoot. Functional tissue T1ρ is highly dependent on the magnetic field strength and experimental parameters such as the power of the spin-locking pulse. With a 500Hz spin-locking pulse, the tissue T1ρ without the blood contribution increased during visual stimulation, but decreased during acidosis-inducing hypercapnia and global ischemia, indicating different signal origins. Phantom studies suggest that it may have contribution from concentration decrease in metabolites. Even though the sensitivity is much weaker than BOLD and its exact interpretation needs further investigation, our results show that non-hemodynamic functional signal can be consistently observed by spin-lock fMRI.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures





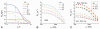
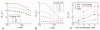
Similar articles
-
Effects of spin-lock field direction on the quantitative measurement of spin-lattice relaxation time constant in the rotating frame (T1ρ) in a clinical MRI system.Med Phys. 2014 Dec;41(12):122301. doi: 10.1118/1.4900607. Med Phys. 2014. PMID: 25471977
-
Improved spatial localization of post-stimulus BOLD undershoot relative to positive BOLD.Neuroimage. 2007 Feb 1;34(3):1084-92. doi: 10.1016/j.neuroimage.2006.10.016. Epub 2006 Dec 11. Neuroimage. 2007. PMID: 17161623 Free PMC article.
-
Exploring the post-stimulus undershoot with spin-echo fMRI: implications for models of neurovascular response.Hum Brain Mapp. 2011 Jan;32(1):141-53. doi: 10.1002/hbm.21003. Hum Brain Mapp. 2011. PMID: 20623748 Free PMC article.
-
Linking brain vascular physiology to hemodynamic response in ultra-high field MRI.Neuroimage. 2018 Mar;168:279-295. doi: 10.1016/j.neuroimage.2017.02.063. Epub 2017 Feb 22. Neuroimage. 2018. PMID: 28254456 Review.
-
Biophysical and physiological origins of blood oxygenation level-dependent fMRI signals.J Cereb Blood Flow Metab. 2012 Jul;32(7):1188-206. doi: 10.1038/jcbfm.2012.23. Epub 2012 Mar 7. J Cereb Blood Flow Metab. 2012. PMID: 22395207 Free PMC article. Review.
Cited by
-
The current status and trend of the functional magnetic resonance combined with stimulation in animals.Front Neurosci. 2022 Sep 23;16:963175. doi: 10.3389/fnins.2022.963175. eCollection 2022. Front Neurosci. 2022. PMID: 36213733 Free PMC article.
-
Development of an MRI biomarker sensitive to tetrameric visual arrestin 1 and its reduction via light-evoked translocation in vivo.FASEB J. 2015 Feb;29(2):554-64. doi: 10.1096/fj.14-254953. Epub 2014 Oct 28. FASEB J. 2015. PMID: 25351983 Free PMC article.
-
New insights into rotating frame relaxation at high field.NMR Biomed. 2016 Sep;29(9):1258-73. doi: 10.1002/nbm.3490. Epub 2016 Feb 11. NMR Biomed. 2016. PMID: 26866422 Free PMC article. Review.
-
Functional MRI using spin lock editing preparation pulses.Magn Reson Imaging. 2014 Sep;32(7):813-8. doi: 10.1016/j.mri.2014.04.001. Epub 2014 Apr 13. Magn Reson Imaging. 2014. PMID: 24848291 Free PMC article.
-
Rapid acquisition strategy for functional T1ρ mapping of the brain.Magn Reson Imaging. 2014 Nov;32(9):1067-77. doi: 10.1016/j.mri.2014.07.010. Epub 2014 Aug 2. Magn Reson Imaging. 2014. PMID: 25093630 Free PMC article.
References
-
- Bandettini PA, Petridou N, Bodurka J. Direct detection of neuronal activity with MRI: Fantasy, possibility, or reality? Applied Magnetic Resonance. 2005;29:65–88.
-
- Borthakur A, Charagundla SR, Wheaton A, Reddy R. T-1ho-weighted MRI using a surface coil to transmit spin-lock pulses. Journal of Magnetic Resonance. 2004;167:306–316. - PubMed
-
- Chu RN, de Zwart JA, van Gelderen P, Fukunaga M, Kellman P, Holroyd T, Duyn JH. Hunting for neuronal currents: absence of rapid MRI signal changes during visual-evoked response. Neuroimage. 2004;23:1059–1067. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

