Beyond oxidative stress: an immunologist's guide to reactive oxygen species
- PMID: 23618831
- PMCID: PMC4250048
- DOI: 10.1038/nri3423
Beyond oxidative stress: an immunologist's guide to reactive oxygen species
Abstract
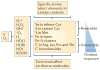
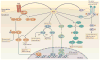
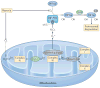
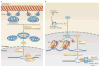
Reactive oxygen species (ROS) react preferentially with certain atoms to modulate functions ranging from cell homeostasis to cell death. Molecular actions include both inhibition and activation of proteins, mutagenesis of DNA and activation of gene transcription. Cellular actions include promotion or suppression of inflammation, immunity and carcinogenesis. ROS help the host to compete against microorganisms and are also involved in intermicrobial competition. ROS chemistry and their pleiotropy make them difficult to localize, to quantify and to manipulate - challenges we must overcome to translate ROS biology into medical advances.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Nathan C, Ding A. Snapshot: reactive oxygen intermediates (ROI) Cell. 2010;140:951–951.e2. - PubMed
-
- Finkel T. From sulfenylation to sulfhydration: what a thiolate needs to tolerate. Sci Signal. 2012;5:pe10. - PubMed
-
- Paul BD, Snyder SH. H2S signalling through protein sulfhydration and beyond. Nature Rev Mol Cell Biol. 2012;13:499–507. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

