The retinoblastoma protein induces apoptosis directly at the mitochondria
- PMID: 23618872
- PMCID: PMC3656319
- DOI: 10.1101/gad.211326.112
The retinoblastoma protein induces apoptosis directly at the mitochondria
Abstract
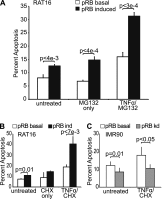
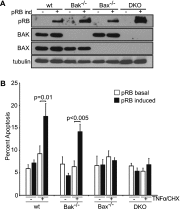
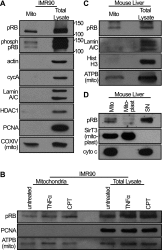
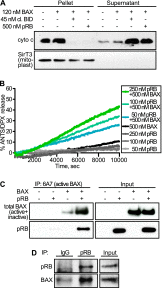
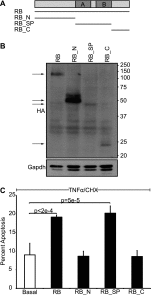
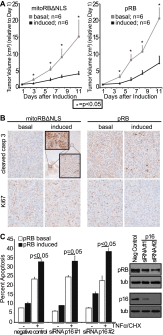
The retinoblastoma protein gene RB-1 is mutated in one-third of human tumors. Its protein product, pRB (retinoblastoma protein), functions as a transcriptional coregulator in many fundamental cellular processes. Here, we report a nonnuclear role for pRB in apoptosis induction via pRB's direct participation in mitochondrial apoptosis. We uncovered this activity by finding that pRB potentiated TNFα-induced apoptosis even when translation was blocked. This proapoptotic function was highly BAX-dependent, suggesting a role in mitochondrial apoptosis, and accordingly, a fraction of endogenous pRB constitutively associated with mitochondria. Remarkably, we found that recombinant pRB was sufficient to trigger the BAX-dependent permeabilization of mitochondria or liposomes in vitro. Moreover, pRB interacted with BAX in vivo and could directly bind and conformationally activate BAX in vitro. Finally, by targeting pRB specifically to mitochondria, we generated a mutant that lacked pRB's classic nuclear roles. This mito-tagged pRB retained the ability to promote apoptosis in response to TNFα and also additional apoptotic stimuli. Most importantly, induced expression of mito-tagged pRB in Rb(-/-);p53(-/-) tumors was sufficient to block further tumor development. Together, these data establish a nontranscriptional role for pRB in direct activation of BAX and mitochondrial apoptosis in response to diverse stimuli, which is profoundly tumor-suppressive.
Keywords: MOMP; apoptosis; cancer; pRB; retinoblastoma protein.
Figures

Comment in
-
RB goes mitochondrial.Genes Dev. 2013 May 1;27(9):975-9. doi: 10.1101/gad.219451.113. Genes Dev. 2013. PMID: 23651852 Free PMC article.
References
-
- Araki K, Ahmad SM, Li G, Bray DA Jr, Saito K, Wang D, Wirtz U, Sreedharan S, O'Malley BW Jr, Li D 2008. Retinoblastoma RB94 enhances radiation treatment of head and neck squamous cell carcinoma. Clin Cancer Res 14: 3514–3519 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
