Androgen receptor inclusions acquire GRP78/BiP to ameliorate androgen-induced protein misfolding stress in embryonic stem cells
- PMID: 23618905
- PMCID: PMC3641345
- DOI: 10.1038/cddis.2013.122
Androgen receptor inclusions acquire GRP78/BiP to ameliorate androgen-induced protein misfolding stress in embryonic stem cells
Abstract
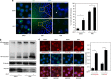
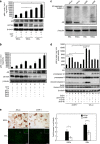
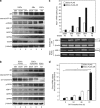
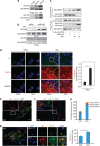
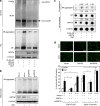
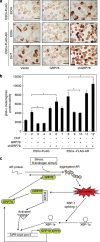
Commitment of differentiating embryonic stem cells (ESCs) toward the various lineages is influenced by many factors, including androgens. However, the mechanisms underlying proteotoxic stress conferred by androgen receptor (AR) actions on embryonic cell fate remains unclear. Here we show that mouse ESCs display stress-related cellular phenotypes in response to androgens during early phase of differentiation. Androgen induced a significant increase in the percentage of ESCs and embryoid bodies with the intranuclear and juxtanuclear AR inclusions, which were colocalized with the E3 ubiquitin ligase, C terminus of Hsc70-interacting protein. Caspase-3 activity corresponded with AR expression, was enhanced in cells engaged more differentiation phenotypes. Androgen-mediated accumulation of AR aggregates exacerbated endoplasmic reticulum (ER) stress and rendered ESCs susceptible to apoptosis. Increasing expression levels of the ER chaperones, GRP78/BiP and GRP94, as well as ER stress markers, such as ATF6, phosphorylated PERK, GADD153/CHOP and spliced XBP-1 mRNA, were dramatically elevated in ESCs overexpressing AR. We found that androgen induced GRP78/BiP to dissociate from ATF6, and act as an AR-interacting protein, which was recruited into AR inclusions in ESCs. GRP78/BiP was also colocalized with AR inclusions in the cells of spinal bulbar muscular atrophy transgenic mouse model. Overexpression of GRP78/BiP suppressed ubiquitination of AR aggregates and ameliorated the misfolded AR-mediated cytopathology in ESCs, whereas knockdown of GRP78/BiP increased the accumulation of AR aggregates and significantly higher levels of caspase-3 activity and cell apoptosis. These results generate novel insight into how ESCs respond to stress induced by misfolded AR proteins and identify GRP78/BiP as a novel regulator of the AR protein quality control.
Figures
Similar articles
-
Androgens modulate autophagy and cell death via regulation of the endoplasmic reticulum chaperone glucose-regulated protein 78/BiP in prostate cancer cells.Cell Death Dis. 2010 Sep 9;1(9):e72. doi: 10.1038/cddis.2010.50. Cell Death Dis. 2010. PMID: 21364676 Free PMC article.
-
CHIP overexpression reduces mutant androgen receptor protein and ameliorates phenotypes of the spinal and bulbar muscular atrophy transgenic mouse model.J Neurosci. 2007 May 9;27(19):5115-26. doi: 10.1523/JNEUROSCI.1242-07.2007. J Neurosci. 2007. PMID: 17494697 Free PMC article.
-
Involvement of androgen receptor and glucose-regulated protein 78 kDa in human hepatocarcinogenesis.Exp Cell Res. 2014 May 1;323(2):326-36. doi: 10.1016/j.yexcr.2014.02.017. Epub 2014 Feb 26. Exp Cell Res. 2014. PMID: 24583399
-
[Therapeutic strategies for Alzheimer disease based on endoplasmic reticulum stress].Nihon Shinkei Seishin Yakurigaku Zasshi. 2010 Aug;30(4):163-8. Nihon Shinkei Seishin Yakurigaku Zasshi. 2010. PMID: 20857693 Review. Japanese.
-
Molecular signal networks and regulating mechanisms of the unfolded protein response.J Zhejiang Univ Sci B. 2017 Jan.;18(1):1-14. doi: 10.1631/jzus.B1600043. J Zhejiang Univ Sci B. 2017. PMID: 28070992 Free PMC article. Review.
Cited by
-
Androgen modulation of Foxp1 and Foxp2 in the developing rat brain: impact on sex specific vocalization.Endocrinology. 2014 Dec;155(12):4881-94. doi: 10.1210/en.2014-1486. Epub 2014 Sep 23. Endocrinology. 2014. PMID: 25247470 Free PMC article.
-
A Crucial Role for the Protein Quality Control System in Motor Neuron Diseases.Front Aging Neurosci. 2020 Jul 21;12:191. doi: 10.3389/fnagi.2020.00191. eCollection 2020. Front Aging Neurosci. 2020. PMID: 32792938 Free PMC article.
-
The androgen receptor as an emerging target in hepatocellular carcinoma.J Hepatocell Carcinoma. 2015 Jun 26;2:91-9. doi: 10.2147/JHC.S48956. eCollection 2015. J Hepatocell Carcinoma. 2015. PMID: 27508198 Free PMC article. Review.
-
The Role of the Protein Quality Control System in SBMA.J Mol Neurosci. 2016 Mar;58(3):348-64. doi: 10.1007/s12031-015-0675-6. Epub 2015 Nov 14. J Mol Neurosci. 2016. PMID: 26572535 Review.
-
Sodium fluoride induces apoptosis and autophagy via the endoplasmic reticulum stress pathway in MC3T3-E1 osteoblastic cells.Mol Cell Biochem. 2019 Apr;454(1-2):77-85. doi: 10.1007/s11010-018-3454-1. Epub 2018 Dec 5. Mol Cell Biochem. 2019. PMID: 30519783
References
-
- Chang CY, Hsuuw YD, Huang FJ, Shyr CR, Chang SY, Huang CK, et al. Androgenic and antiandrogenic effects and expression of androgen receptor in mouse embryonic stem cells. Fertil Steril. 2006;85 (Suppl 1:1195–1203. - PubMed
-
- Rosenberg KM, Sherman GF. Testosterone induced pup-killing behavior in the ovariectomized female rat. Physiol Behav. 1974;13:697–699. - PubMed
-
- Mann MA, Svare B. Prenatal testosterone exposure elevates maternal aggression in mice. Physiol Behav. 1983;30:503–507. - PubMed
-
- Fritz H, Giese K, Suter HP. Prenatal and postnatal development of rats following the maternal treatment with testosterone during the late period of embryogenesis. Arzneim Forsch. 1984;34:780–782. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

