NMDA receptor subunit composition determines beta-amyloid-induced neurodegeneration and synaptic loss
- PMID: 23618906
- PMCID: PMC3641351
- DOI: 10.1038/cddis.2013.129
NMDA receptor subunit composition determines beta-amyloid-induced neurodegeneration and synaptic loss
Abstract
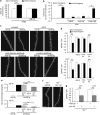
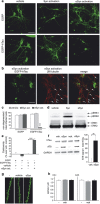
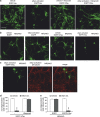
Aggregates of amyloid-beta (Aβ) and tau are hallmarks of Alzheimer's disease (AD) leading to neurodegeneration and synaptic loss. While increasing evidence suggests that inhibition of N-methyl-D-aspartate receptors (NMDARs) may mitigate certain aspects of AD neuropathology, the precise role of different NMDAR subtypes for Aβ- and tau-mediated toxicity remains to be elucidated. Using mouse organotypic hippocampal slice cultures from arcAβ transgenic mice combined with Sindbis virus-mediated expression of human wild-type tau protein (hTau), we show that Aβ caused dendritic spine loss independently of tau. However, the presence of hTau was required for Aβ-induced cell death accompanied by increased hTau phosphorylation. Inhibition of NR2B-containing NMDARs abolished Aβ-induced hTau phosphorylation and toxicity by preventing GSK-3β activation but did not affect dendritic spine loss. Inversely, NR2A-containing NMDAR inhibition as well as NR2A-subunit knockout diminished dendritic spine loss but not the Aβ effect on hTau. Activation of extrasynaptic NMDARs in primary neurons caused degeneration of hTau-expressing neurons, which could be prevented by NR2B-NMDAR inhibition but not by NR2A knockout. Furthermore, caspase-3 activity was increased in arcAβ transgenic cultures. Activity was reduced by NR2A knockout but not by NR2B inhibition. Accordingly, caspase-3 inhibition abolished spine loss but not hTau-dependent toxicity in arcAβ transgenic slice cultures. Our data show that Aβ induces dendritic spine loss via a pathway involving NR2A-containing NMDARs and active caspase-3 whereas activation of eSyn NR2B-containing NMDARs is required for hTau-dependent neurodegeneration, independent of caspase-3.
Figures


References
-
- Roberson E, Scearce-Levie K, Palop JJ, Yan F, Cheng IH, Wu T, et al. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science. 2007;31:750–754. - PubMed
-
- Ittner LM, Ke YD, Delerue F, Bi M, Gladbach A, van Eersel J, et al. Dendritic function of tau mediates amyloid-beta toxicity in Alzheimer's disease mouse models. Cell. 2010;142:387–397. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

