Refinement of the Listeria monocytogenes σB regulon through quantitative proteomic analysis
- PMID: 23618998
- PMCID: PMC3709693
- DOI: 10.1099/mic.0.066001-0
Refinement of the Listeria monocytogenes σB regulon through quantitative proteomic analysis
Abstract
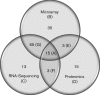
σ(B) is an alternative σ factor that regulates stress response and virulence genes in the foodborne pathogen Listeria monocytogenes. To gain further insight into σ(B)-dependent regulatory mechanisms in L. monocytogenes, we (i) performed quantitative proteomic comparisons between the L. monocytogenes parent strain 10403S and an isogenic ΔsigB mutant and (ii) conducted a meta-analysis of published microarray studies on the 10403S σ(B) regulon. A total of 134 genes were found to be significantly positively regulated by σ(B) at the transcriptomic level with >75 % of these genes preceded by putative σ(B)-dependent promoters; 21 of these 134 genes were also found to be positively regulated by σ(B) through proteomics. In addition, 15 proteins were only found to be positively regulated by σ(B) through proteomics analyses, including Lmo1349, a putative glycine cleavage system protein. The lmo1349 gene is preceded by a 5' UTR that functions as a glycine riboswitch, which suggests regulation of glycine metabolism by σ(B) in L. monocytogenes. Herein, we propose a model where σ(B) upregulates pathways that facilitate biosynthesis and uptake of glycine, which may then activate this riboswitch. Our data also (i) identified a number of σ(B)-dependent proteins that appear to be encoded by genes that are co-regulated by multiple transcriptional regulators, in particular PrfA, and (ii) found σ(B)-dependent genes and proteins to be overrepresented in the 'energy metabolism' role category, highlighting contributions of the σ(B) regulon to L. monocytogenes energy metabolism as well as a role of PrfA and σ(B) interaction in regulating aspects of energy metabolism in L. monocytogenes.
Figures


Similar articles
-
Transcriptomic and phenotypic analyses identify coregulated, overlapping regulons among PrfA, CtsR, HrcA, and the alternative sigma factors sigmaB, sigmaC, sigmaH, and sigmaL in Listeria monocytogenes.Appl Environ Microbiol. 2011 Jan;77(1):187-200. doi: 10.1128/AEM.00952-10. Epub 2010 Oct 29. Appl Environ Microbiol. 2011. PMID: 21037293 Free PMC article.
-
Contributions of σ(B) and PrfA to Listeria monocytogenes salt stress under food relevant conditions.Int J Food Microbiol. 2014 May 2;177:98-108. doi: 10.1016/j.ijfoodmicro.2014.02.018. Epub 2014 Mar 3. Int J Food Microbiol. 2014. PMID: 24631633 Free PMC article.
-
The Listeria monocytogenes σB regulon and its virulence-associated functions are inhibited by a small molecule.mBio. 2011 Nov 29;2(6):e00241-11. doi: 10.1128/mBio.00241-11. Print 2011. mBio. 2011. PMID: 22128349 Free PMC article.
-
Systematic review of the Listeria monocytogenes σB regulon supports a role in stress response, virulence and metabolism.Future Microbiol. 2019 Jun;14:801-828. doi: 10.2217/fmb-2019-0072. Epub 2019 Jul 4. Future Microbiol. 2019. PMID: 31271064
-
Cross Talk between SigB and PrfA in Listeria monocytogenes Facilitates Transitions between Extra- and Intracellular Environments.Microbiol Mol Biol Rev. 2019 Sep 4;83(4):e00034-19. doi: 10.1128/MMBR.00034-19. Print 2019 Nov 20. Microbiol Mol Biol Rev. 2019. PMID: 31484692 Free PMC article. Review.
Cited by
-
Redefining the Clostridioides difficile σB Regulon: σB Activates Genes Involved in Detoxifying Radicals That Can Result from the Exposure to Antimicrobials and Hydrogen Peroxide.mSphere. 2020 Sep 16;5(5):e00728-20. doi: 10.1128/mSphere.00728-20. mSphere. 2020. PMID: 32938698 Free PMC article.
-
Listeria monocytogenes Pathogenesis: The Role of Stress Adaptation.Microorganisms. 2022 Jul 27;10(8):1522. doi: 10.3390/microorganisms10081522. Microorganisms. 2022. PMID: 36013940 Free PMC article. Review.
-
An advanced bioinformatics approach for analyzing RNA-seq data reveals sigma H-dependent regulation of competence genes in Listeria monocytogenes.BMC Genomics. 2016 Feb 16;17:115. doi: 10.1186/s12864-016-2432-9. BMC Genomics. 2016. PMID: 26880300 Free PMC article.
-
Chitinase Expression in Listeria monocytogenes Is Influenced by lmo0327, Which Encodes an Internalin-Like Protein.Appl Environ Microbiol. 2017 Oct 31;83(22):e01283-17. doi: 10.1128/AEM.01283-17. Print 2017 Nov 15. Appl Environ Microbiol. 2017. PMID: 28887418 Free PMC article.
-
Transcriptomic and Phenotypic Analyses of the Sigma B-Dependent Characteristics and the Synergism between Sigma B and Sigma L in Listeria monocytogenes EGD-e.Microorganisms. 2020 Oct 23;8(11):1644. doi: 10.3390/microorganisms8111644. Microorganisms. 2020. PMID: 33114171 Free PMC article.
References
-
- Abram F., Su W. L., Wiedmann M., Boor K. J., Coote P., Botting C., Karatzas K. A., O’Byrne C. P. (2008a). Proteomic analyses of a Listeria monocytogenes mutant lacking σB identify new components of the σB regulon and highlight a role for σB in the utilization of glycerol. Appl Environ Microbiol 74, 594–604. 10.1128/AEM.01921-07 - DOI - PMC - PubMed
-
- Abram F., Starr E., Karatzas K. A., Matlawska-Wasowska K., Boyd A., Wiedmann M., Boor K. J., Connally D., O’Byrne C. P. (2008b). Identification of components of the sigma B regulon in Listeria monocytogenes that contribute to acid and salt tolerance. Appl Environ Microbiol 74, 6848–6858. 10.1128/AEM.00442-08 - DOI - PMC - PubMed
-
- Bennett H. J., Pearce D. M., Glenn S., Taylor C. M., Kuhn M., Sonenshein A. L., Andrew P. W., Roberts I. S. (2007). Characterization of relA and codY mutants of Listeria monocytogenes: identification of the CodY regulon and its role in virulence. Mol Microbiol 63, 1453–1467. 10.1111/j.1365-2958.2007.05597.x - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

