Uterine glands: development, function and experimental model systems
- PMID: 23619340
- PMCID: PMC3749806
- DOI: 10.1093/molehr/gat031
Uterine glands: development, function and experimental model systems
Abstract
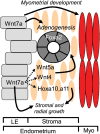
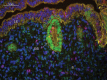
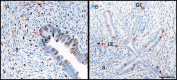
Development of uterine glands (adenogenesis) in mammals typically begins during the early post-natal period and involves budding of nascent glands from the luminal epithelium and extensive cell proliferation in these structures as they grow into the surrounding stroma, elongate and mature. Uterine glands are essential for pregnancy, as demonstrated by the infertility that results from inhibiting the development of these glands through gene mutation or epigenetic strategies. Several genes, including forkhead box A2, beta-catenin and members of the Wnt and Hox gene families, are implicated in uterine gland development. Progestins inhibit uterine epithelial proliferation, and this has been employed as a strategy to develop a model in which progestin treatment of ewes for 8 weeks from birth produces infertile adults lacking uterine glands. More recently, mouse models have been developed in which neonatal progestin treatment was used to permanently inhibit adenogenesis and adult fertility. These studies revealed a narrow and well-defined window in which progestin treatments induced permanent infertility by impairing neonatal gland development and establishing endometrial changes that result in implantation defects. These model systems are being utilized to better understand the molecular mechanisms underlying uterine adenogenesis and endometrial function. The ability of neonatal progestin treatment in sheep and mice to produce infertility suggests that an approach of this kind may provide a contraceptive strategy with application in other species. Recent studies have defined the temporal patterns of adenogenesis in uteri of neonatal and juvenile dogs and work is underway to determine whether neonatal progestin or other steroid hormone treatments might be a viable contraceptive approach in this species.
Keywords: adenogenesis; contraception; endometrium; murine; ungulate.
Figures

References
-
- Amoroso EC. Placentation. In: Parks AS, editor. Marshall's Physiology of Reproduction No. 2. London: Longmans, Grene and Co., Ltd.; 1952.
-
- Arango NA, Szotek PP, Manganaro TF, Oliva E, Donahoe PK, Teixeira J. Conditional deletion of beta-catenin in the mesenchyme of the developing mouse uterus results in a switch to adipogenesis in the myometrium. Dev Biol. 2005;288:276–283. - PubMed
-
- Atkinson BA, King GJ, Amoroso EC. Development of the caruncular and intercaruncular regions in the bovine endometrium. Biol Reprod. 1984;30:763–774. doi:10.1095/biolreprod30.3.763. - DOI - PubMed
-
- Bal HS, Getty R. Postnatal growth of the swine uterus from birth to six months. Growth. 1970;34:15–30. - PubMed
-
- Bartlett PC, Bartlett A, Walshaw S, Halstead S. Rates of euthanasia and adoption for dogs and cats in Michigan animal shelters. J Appl Anim Welfare Sci. 2005;8:97–104. doi:10.1207/s15327604jaws0802_2. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

