MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy
- PMID: 23619365
- PMCID: PMC3638905
- DOI: 10.1172/JCI64365
MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy
Abstract
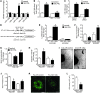
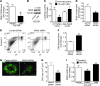
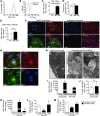
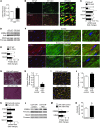
Peripartum cardiomyopathy (PPCM) is a life-threatening pregnancy-associated cardiomyopathy in previously healthy women. Although PPCM is driven in part by the 16-kDa N-terminal prolactin fragment (16K PRL), the underlying molecular mechanisms are poorly understood. We found that 16K PRL induced microRNA-146a (miR-146a) expression in ECs, which attenuated angiogenesis through downregulation of NRAS. 16K PRL stimulated the release of miR-146a-loaded exosomes from ECs. The exosomes were absorbed by cardiomyocytes, increasing miR-146a levels, which resulted in a subsequent decrease in metabolic activity and decreased expression of Erbb4, Notch1, and Irak1. Mice with cardiomyocyte-restricted Stat3 knockout (CKO mice) exhibited a PPCM-like phenotype and displayed increased cardiac miR-146a expression with coincident downregulation of Erbb4, Nras, Notch1, and Irak1. Blocking miR-146a with locked nucleic acids or antago-miRs attenuated PPCM in CKO mice without interrupting full-length prolactin signaling, as indicated by normal nursing activities. Finally, miR-146a was elevated in the plasma and hearts of PPCM patients, but not in patients with dilated cardiomyopathy. These results demonstrate that miR-146a is a downstream-mediator of 16K PRL that could potentially serve as a biomarker and therapeutic target for PPCM.
Figures

Comment in
-
A microRNA links prolactin to peripartum cardiomyopathy.J Clin Invest. 2013 May;123(5):1925-7. doi: 10.1172/JCI69286. Epub 2013 Apr 24. J Clin Invest. 2013. PMID: 23619357 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

