Visually guided gradation of prey capture movements in larval zebrafish
- PMID: 23619412
- PMCID: PMC4074221
- DOI: 10.1242/jeb.087742
Visually guided gradation of prey capture movements in larval zebrafish
Abstract
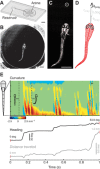
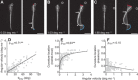
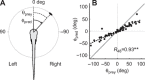
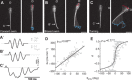
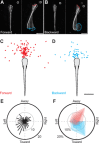
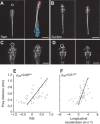
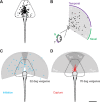
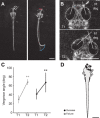
A mechanistic understanding of goal-directed behavior in vertebrates is hindered by the relative inaccessibility and size of their nervous systems. Here, we have studied the kinematics of prey capture behavior in a highly accessible vertebrate model organism, the transparent larval zebrafish (Danio rerio), to assess whether they use visual cues to systematically adjust their movements. We found that zebrafish larvae scale the speed and magnitude of turning movements according to the azimuth of one of their standard prey, paramecia. They also bias the direction of subsequent swimming movements based on prey azimuth and select forward or backward movements based on the prey's direction of travel. Once within striking distance, larvae generate either ram or suction capture behaviors depending on their distance from the prey. From our experimental estimations of ocular receptive fields, we ascertained that the ultimate decision to consume prey is likely a function of the progressive vergence of the eyes that places the target in a proximal binocular 'capture zone'. By repeating these experiments in the dark, we demonstrate that paramecia are only consumed if they contact the anterior extremities of larvae, which triggers ocular vergence and tail movements similar to close proximity captures in lit conditions. These observations confirm the importance of vision in the graded movements we observe leading up to capture of more distant prey in the light, and implicate somatosensation in captures in the absence of light. We discuss the implications of these findings for future work on the neural control of visually guided behavior in zebrafish.
Keywords: Danio rerio; behavior; kinematics; visuomotor integration.
Figures

References
-
- Angeles Luque M., Pilar Pérez-Pérez M., Herrero L., Torres B. (2005). Involvement of the optic tectum and mesencephalic reticular formation in the generation of saccadic eye movements in goldfish. Brain Res. Brain Res. Rev. 49, 388-397 - PubMed
-
- Baier H., Klostermann S., Trowe T., Karlstrom R. O., Nüsslein-Volhard C., Bonhoeffer F. (1996). Genetic dissection of the retinotectal projection. Development 123, 415-425 - PubMed
-
- Bastian J. (1982). Vision and electroreception - integration of sensory information in the optic tectum of the weakly electric fish Apteronotus albifrons. J. Comp. Physiol. 147, 287-297
-
- Bhatt D. H., McLean D. L., Hale M. E., Fetcho J. R. (2007). Grading movement strength by changes in firing intensity versus recruitment of spinal interneurons. Neuron 53, 91-102 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

