The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo
- PMID: 23619421
- PMCID: PMC3695748
- DOI: 10.1093/cvr/cvt101
The atherosusceptible endothelium: endothelial phenotypes in complex haemodynamic shear stress regions in vivo
Abstract
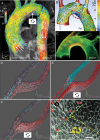
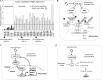
Atherosclerosis initiates at predictable focal sites and develops to a spatially regional disease with limited distribution. There is compelling evidence that links haemodynamics to the localized origin of atherosclerotic lesions. Arterial flow in vivo is unsteady, dynamically complex, and regionally variable. Sites susceptible to atherosclerosis near arterial branches and curves are associated with regions of disturbed blood flow that contain repetitive phases of flow reversal resulting in steep multidirectional temporal and spatial gradients of wall shear stresses. Endothelium in atherosusceptible regions relative to protected sites shows activation of endoplasmic reticulum (ER) stress and the unfolded protein response (UPR), the altered expression of pro-inflammatory Nuclear Factor kappa B (NFκB) and oxidant/antioxidant pathways, and low expression of major protective factors, notably endothelial nitric oxide synthase and Kruppel-like Factors KLF2 and KLF4. At some atherosusceptible locations, reactive oxygen species levels are significantly elevated. Here we describe flow-related phenotypes identified in steady-state in vivo and outline some of the molecular mechanisms that may contribute to pre-lesional atherosusceptibility as deduced from complementary cell experiments in vitro. We conclude that disturbed flow is a significant local risk factor for atherosclerosis that induces a chronic low-level inflammatory state, an adaptive response to ensure continued function at the expense of increased susceptibility to atherogenesis. Surprisingly, when challenged by short-term hypercholesterolaemia in vivo, atherosusceptible endothelial phenotype was resistant to greater pro-inflammatory expression, suggesting that sustained hyperlipidaemia is required to overcome these protective characteristics.
Keywords: Atherosclerosis; Endothelial phenotype; Genomicsv; Haemodynamics; Inflammation.
Figures



References
-
- Flaherty JT, Pierce JE, Ferrans VJ, Patel DJ, Tucker WK, Fry DL. Endothelial nuclear patterns in the canine arterial tree with particular reference to hemodynamic events. Circ. Res. 1972;30:23–33. doi:10.1161/01.RES.30.1.23. - DOI - PubMed
-
- Dewey CF, Gimbrone MA, Bussolari SR, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Engineer. 1981;103:177–185. doi:10.1115/1.3138276. - DOI - PubMed
-
- Passerini AG, Polacek DC, Shi C, Francesco NM, Manduchi E, Grant GR, et al. Coexisting pro-inflammatory and anti-oxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci USA. 2004;101:2482–2487. doi:10.1073/pnas.0305938101. - DOI - PMC - PubMed
-
- Civelek M, Manduchi E, Riley RJ, Stoeckert CJ, Davies PF. Coronary artery endothelial transcriptome in vivo: identification of ER-stress and enhanced ROS by gene connectivity network analysis. Circ Cardiovasc Genet. 2011;4:243–252. doi:10.1161/CIRCGENETICS.110.958926. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

