Characterization and quantification of endogenous fatty acid nitroalkene metabolites in human urine
- PMID: 23620137
- PMCID: PMC3679401
- DOI: 10.1194/jlr.M037804
Characterization and quantification of endogenous fatty acid nitroalkene metabolites in human urine
Abstract
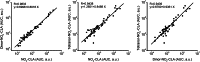
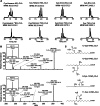
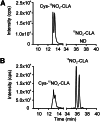
The oxidation and nitration of unsaturated fatty acids transforms cell membrane and lipoprotein constituents into mediators that regulate signal transduction. The formation of 9-NO2-octadeca-9,11-dienoic acid and 12-NO2-octadeca-9,11-dienoic acid stems from peroxynitrite- and myeloperoxidase-derived nitrogen dioxide reactions as well as secondary to nitrite disproportionation under the acidic conditions of digestion. Broad anti-inflammatory and tissue-protective responses are mediated by nitro-fatty acids. It is now shown that electrophilic fatty acid nitroalkenes are present in the urine of healthy human volunteers (9.9 ± 4.0 pmol/mg creatinine); along with electrophilic 16- and 14-carbon nitroalkenyl β-oxidation metabolites. High resolution mass determinations and coelution with isotopically-labeled metabolites support renal excretion of cysteine-nitroalkene conjugates. These products of Michael addition are in equilibrium with the free nitroalkene pool in urine and are displaced by thiol reaction with mercury chloride. This reaction increases the level of free nitroalkene fraction >10-fold and displays a K(D) of 7.5 × 10(-6) M. In aggregate, the data indicates that formation of Michael adducts by electrophilic fatty acids is favored under biological conditions and that reversal of these addition reactions is critical for detecting both parent nitroalkenes and their metabolites. The measurement of this class of mediators can constitute a sensitive noninvasive index of metabolic and inflammatory status.
Keywords: Michael addition; electrophile; nitration; nitro-fatty acid.
Figures





References
-
- Chiang N., Serhan C. N., Dahlen S. E., Drazen J. M., Hay D. W. P., Rovati G. E., Shimizu T., Yokomizo T., Brink C. 2006. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol. Rev. 58: 463–487 - PubMed
-
- Kansanen E., Jyrkkanen H. K., Volger O. L., Leinonen H., Kivela A. M., Hakkinen S. K., Woodcock S. R., Schopfer F. J., Horrevoets A. J., Yla-Herttuala S., et al. 2009. Nrf2-dependent and -independent responses to nitro-fatty acids in human endothelial cells: identification of heat shock response as the major pathway activated by nitro-oleic acid. J. Biol. Chem. 284: 33233–33241 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL132550/HL/NHLBI NIH HHS/United States
- P30 DK072506/DK/NIDDK NIH HHS/United States
- R01-HL-058115/HL/NHLBI NIH HHS/United States
- P30-DK-072506/DK/NIDDK NIH HHS/United States
- R01-HL-64937/HL/NHLBI NIH HHS/United States
- R01-AT-006822-01/AT/NCCIH NIH HHS/United States
- P01-HL-103455/HL/NHLBI NIH HHS/United States
- P01 HL103455/HL/NHLBI NIH HHS/United States
- R37 HL058115/HL/NHLBI NIH HHS/United States
- R01 HL058115/HL/NHLBI NIH HHS/United States
- R01 AT006822/AT/NCCIH NIH HHS/United States
- R01 HL064937/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

