Missense mutation in mouse GALC mimics human gene defect and offers new insights into Krabbe disease
- PMID: 23620143
- PMCID: PMC3736866
- DOI: 10.1093/hmg/ddt190
Missense mutation in mouse GALC mimics human gene defect and offers new insights into Krabbe disease
Abstract
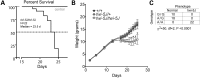
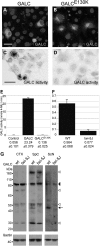
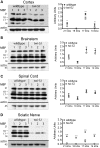
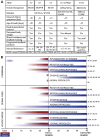
Krabbe disease is a devastating pediatric leukodystrophy caused by mutations in the galactocerebrosidase (GALC) gene. A significant subset of the infantile form of the disease is due to missense mutations that result in aberrant protein production. The currently used mouse model, twitcher, has a nonsense mutation not found in Krabbe patients, although it is similar to the human 30 kb deletion in abrogating GALC expression. Here, we identify a spontaneous mutation in GALC, GALCtwi-5J, that precisely matches the E130K missense mutation in patients with infantile Krabbe disease. GALCtwi-5J homozygotes show loss of enzymatic activity despite normal levels of precursor protein, and manifest a more severe phenotype than twitcher, with half the life span. Although neuropathological hallmarks such as gliosis, globoid cells and psychosine accumulation are present throughout the nervous system, the CNS does not manifest significant demyelination. In contrast, the PNS is severely hypomyelinated and lacks large diameter axons, suggesting primary dysmyelination, rather than a demyelinating process. Our data indicate that early demise is due to mechanisms other than myelin loss and support an important role for neuroinflammation in Krabbe disease progression. Furthermore, our results argue against a causative relationship between psychosine accumulation, white matter loss and gliosis.
Figures







References
-
- Igisu H., Suzuki K. Progressive accumulation of toxic metabolite in a genetic leukodystrophy. Science. 1984;224:753–755. doi:10.1126/science.6719111. - DOI - PubMed
-
- Xu Y.H., Barnes S., Sun Y., Grabowski G.A. Multi-system disorders of glycosphingolipid and ganglioside metabolism. J. Lipid Res. 2010;51:1643–1675. doi:10.1194/jlr.R003996. - DOI - PMC - PubMed
-
- Jacobs J.M., Scaravilli F., De Aranda F.T. The pathogenesis of globoid cell leucodystrophy in peripheral nerve of the mouse mutant twitcher. J. Neurol. Sci. 1982;55:285–304. doi:10.1016/0022-510X(82)90127-7. - DOI - PubMed
-
- Suzuki K., Taniike M. Murine model of genetic demyelinating disease: the twitcher mouse. Microsc. Res. Tech. 1995;32:204–214. doi:10.1002/jemt.1070320304. - DOI - PubMed
-
- Wenger D.A. 1997. Krabbe disease. GeneReviews at GeneTests: Medical Genetics Information Resource (database online). Copyright, University of Washington, Seattle, 2010.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

