Nephrotic syndrome caused by immune-mediated acquired LCAT deficiency
- PMID: 23620397
- PMCID: PMC3736715
- DOI: 10.1681/ASN.2012090913
Nephrotic syndrome caused by immune-mediated acquired LCAT deficiency
Abstract
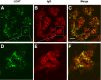
Lecithin-cholesterol acyltransferase (LCAT) is an enzyme involved in maintaining cholesterol homeostasis. In familial LCAT deficiency (FLD), abnormal lipid deposition causes renal injury and nephrotic syndrome, frequently progressing to ESRD. Here, we describe a 63-year-old Japanese woman with no family history of renal disease who presented with nephrotic syndrome. The laboratory data revealed an extremely low level of serum HDL and undetectable serum LCAT activity. Renal biopsy showed glomerular lipid deposition with prominent accumulation of foam cells, similar to the histologic findings of FLD. In addition, she had subepithelial electron-dense deposits compatible with membranous nephropathy, which are not typical of FLD. A mixing test and coimmunoprecipitation study demonstrated the presence of an inhibitory anti-LCAT antibody in the patient's serum. Immunohistochemistry and immunofluorescence detected LCAT along parts of the glomerular capillary walls, suggesting that LCAT was an antigen responsible for the membranous nephropathy. Treatment with steroids resulted in complete remission of the nephrotic syndrome, normalization of serum LCAT activity and HDL level, and disappearance of foam cell accumulation in renal tissue. In summary, inhibitory anti-LCAT antibody can lead to glomerular lesions similar to those observed in FLD.
Figures





References
-
- Jonas A: Lecithin cholesterol acyltransferase. Biochim Biophys Acta 1529: 245–256, 2000 - PubMed
-
- Zannis VI, Chroni A, Krieger M: Role of apoA-I, ABCA1, LCAT, and SR-BI in the biogenesis of HDL. J Mol Med (Berl) 84: 276–294, 2006 - PubMed
-
- Calabresi L, Franceschini G: Lecithin:cholesterol acyltransferase, high-density lipoproteins, and atheroprotection in humans. Trends Cardiovasc Med 20: 50–53, 2010 - PubMed
-
- Gjone E, Norum KR: Familial serum cholesterol ester deficiency. Clinical study of a patient with a new syndrome. Acta Med Scand 183: 107–112, 1968 - PubMed
-
- Carlson LA, Philipson B: Fish-eye disease. A new familial condition with massive corneal opacities and dyslipoproteinaemia. Lancet 2: 922–924, 1979 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

