Histone deacetylase inhibitor enhances recovery after AKI
- PMID: 23620402
- PMCID: PMC3665399
- DOI: 10.1681/ASN.2012111055
Histone deacetylase inhibitor enhances recovery after AKI
Abstract
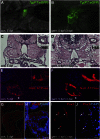
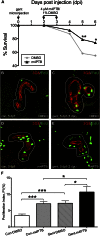
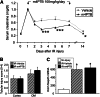
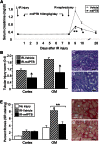
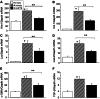
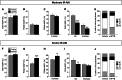
At present, there are no effective therapies to ameliorate injury, accelerate recovery, or prevent postinjury fibrosis after AKI. Here, we sought to identify candidate compounds that accelerate recovery after AKI by screening for small molecules that increase proliferation of renal progenitor cells in zebrafish embryos. One compound identified from this screen was the histone deacetylase inhibitor methyl-4-(phenylthio)butanoate, which we subsequently administered to zebrafish larvae and mice 24-48 hours after inducing AKI. In zebrafish, treatment with the compound increased larval survival and proliferation of renal tubular epithelial cells. In mice, treatment accelerated recovery, reduced postinjury tubular atrophy and interstitial fibrosis, and increased the regenerative capacity of actively cycling renal tubular cells by decreasing the number of cells in G2/M arrest. These data suggest that accelerating recovery may be a viable approach to treating AKI and provide proof of concept that a screen in zebrafish embryos can identify therapeutic candidates for kidney injury.
Figures

References
-
- Lameire N, Van Biesen W, Vanholder R: The changing epidemiology of acute renal failure. Nat Clin Pract Nephrol 2: 364–377, 2006 - PubMed
-
- Waikar SS, Liu KD, Chertow GM: Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol 3: 844–861, 2008 - PubMed
-
- Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C, Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 2R01 HD053287/HD/NICHD NIH HHS/United States
- P30 DK079341/DK/NIDDK NIH HHS/United States
- P30 DK079307/DK/NIDDK NIH HHS/United States
- 1R01 HL093057/HL/NHLBI NIH HHS/United States
- R01 DK069403/DK/NIDDK NIH HHS/United States
- R01 HL093057/HL/NHLBI NIH HHS/United States
- 1RC4DK090770/DK/NIDDK NIH HHS/United States
- 1RC4 DK090770/DK/NIDDK NIH HHS/United States
- 2R01 DK069403/DK/NIDDK NIH HHS/United States
- R01 HD053287/HD/NICHD NIH HHS/United States
- 1P30DK079341/DK/NIDDK NIH HHS/United States
- 1P30DK079307/DK/NIDDK NIH HHS/United States
- RC4 DK090770/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

