Clinical activity and safety of combination therapy with temsirolimus and bevacizumab for advanced melanoma: a phase II trial (CTEP 7190/Mel47)
- PMID: 23620404
- PMCID: PMC3700572
- DOI: 10.1158/1078-0432.CCR-12-3919
Clinical activity and safety of combination therapy with temsirolimus and bevacizumab for advanced melanoma: a phase II trial (CTEP 7190/Mel47)
Abstract
Purpose: A CTEP-sponsored phase II trial was conducted to evaluate safety and clinical activity of combination therapy with CCI-779 (temsirolimus) and bevacizumab in patients with advanced melanoma.
Experimental design: Patients with unresectable stage III to IV melanoma were treated intravenously with temsirolimus 25 mg weekly and bevacizumab 10 mg every 2 weeks. Adverse events were recorded using CTCAE v3.0. Tumor response was assessed by Response Evaluation Criteria in Solid Tumors and overall survival was recorded. Correlative studies measured protein kinases and histology of tumor biopsies and immune function in peripheral blood.
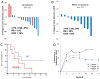
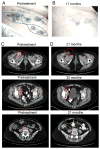
Results: Seventeen patients were treated. Most patients tolerated treatment well, but 2 had grade 4 lymphopenia and 1 developed reversible grade 2 leukoencephalopathy. Best clinical response was partial response (PR) in 3 patients [17.7%, 90% confidence interval (CI) 5, 0-39.6], stable disease at 8 weeks (SD) in 9 patients, progressive disease (PD) in 4 patients, and not evaluable in 1 patient. Maximal response duration for PR was 35 months. Ten evaluable patients had BRAF(WT) tumors, among whom 3 had PRs, 5 had SD, and 2 had PD. Correlative studies of tumor biopsies revealed decreased phospho-S6K (d2 and d23 vs. d1, P < 0.001), and decreased mitotic rate (Ki67(+)) among melanoma cells by d23 (P = 0.007). Effects on immune functions were mixed, with decreased alloreactive T-cell responses and decreased circulating CD4(+)FoxP3(+) cells.
Conclusion: These data provide preliminary evidence for clinical activity of combination therapy with temsirolimus and bevacizumab, which may be greater in patients with BRAF(wt) melanoma. Mixed effects on immunologic function also support combination with immune therapies.
©2013 AACR.
Conflict of interest statement
The authors declare that there are no conflicts of interest or financial interests.
Figures



References
-
- Ko JM, Fisher DE. A new era: melanoma genetics and therapeutics. J Pathol. 2011;223:241–50. - PubMed
-
- Huang S, Houghton PJ. Targeting mTOR signaling for cancer therapy. Curr Opin Pharmacol. 2003;3:371–7. - PubMed
-
- Stahl JM, Sharma A, Cheung M, Zimmerman M, Cheng JQ, Bosenberg MW, et al. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res. 2004;64:7002–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

