The effect of breast cancer resistance protein, multidrug resistant protein 1, and organic anion-transporting polypeptide 1B3 on the antitumor efficacy of the lipophilic camptothecin 7-t-butyldimethylsilyl-10-hydroxycamptothecin (AR-67) in vitro
- PMID: 23620484
- PMCID: PMC3684821
- DOI: 10.1124/dmd.112.050021
The effect of breast cancer resistance protein, multidrug resistant protein 1, and organic anion-transporting polypeptide 1B3 on the antitumor efficacy of the lipophilic camptothecin 7-t-butyldimethylsilyl-10-hydroxycamptothecin (AR-67) in vitro
Abstract
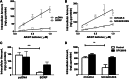
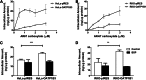
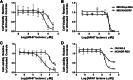
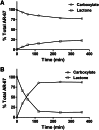
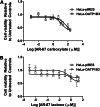
AR-67 (7-t-butyldimethylsilyl-10-hydroxycamptothecin) is a lipophilic camptothecin analog, currently under early stage clinical trials. Transporters are known to have an impact on the disposition of camptothecins and on the response to chemotherapeutics in general due to their expression in tumor tissues. Therefore, we investigated the interplay between the breast cancer resistance protein (BCRP), multidrug resistant protein 1 (MDR1), and organic anion-transporting polypeptide (OATP) 1B1/1B3 transporters and AR-67 and their impact on the toxicity profile of AR-67. Using cell lines expressing the aforementioned transporters, we showed that the lipophilic AR-67 lactone form is a substrate for efflux transporters BCRP and MDR1. Additionally, OATP1B1 and OATP1B3 facilitated the uptake of AR-67 carboxylate in SLCO1B1- and SLCO1B3-transfected cell systems compared with the mock-transfected ones. Notably, both BCRP and MDR1 conferred resistance to AR-67 lactone. Prompted by recent studies showing increased OATP1B3 expression in certain cancer types, we investigated the effect of OATP1B3 expression on cell viability after exposure to AR-67 carboxylate. OATP1B3-expressing cells had increased carboxylate uptake as compared with mock-transfected cells but were not sensitized because the intracellular amount of lactone was 50-fold higher than that of carboxylate and comparable between OATP1B3-expressing and OATP1B3-nonexpressing cells. In conclusion, BCRP- and MDR1-mediated efflux of AR-67 lactone confers resistance to AR-67, but OATP1B3-mediated uptake of the AR-67 carboxylate does not sensitize OATP1B3-expressing tumor cells.
Figures




References
-
- Abe T, Kakyo M, Tokui T, Nakagomi R, Nishio T, Nakai D, Nomura H, Unno M, Suzuki M, Naitoh T, et al. (1999) Identification of a novel gene family encoding human liver-specific organic anion transporter LST-1. J Biol Chem 274:17159–17163 - PubMed
-
- Abe T, Unno M, Onogawa T, Tokui T, Kondo TN, Nakagomi R, Adachi H, Fujiwara K, Okabe M, Suzuki T, et al. (2001) LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers. Gastroenterology 120:1689–1699 - PubMed
-
- Adane ED, Liu Z, Xiang TX, Anderson BD, Leggas M. (2010) Factors affecting the in vivo lactone stability and systemic clearance of the lipophilic camptothecin analogue AR-67. Pharm Res 27:1416–1425 - PubMed
-
- Bom D, Curran DP, Kruszewski S, Zimmer SG, Thompson Strode J, Kohlhagen G, Du W, Chavan AJ, Fraley KA, Bingcang AL, et al. (2000) The novel silatecan 7-tert-butyldimethylsilyl-10-hydroxycamptothecin displays high lipophilicity, improved human blood stability, and potent anticancer activity. J Med Chem 43:3970–3980 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

