Inhibition of CYP2C19 and CYP3A4 by omeprazole metabolites and their contribution to drug-drug interactions
- PMID: 23620487
- PMCID: PMC3684819
- DOI: 10.1124/dmd.113.051722
Inhibition of CYP2C19 and CYP3A4 by omeprazole metabolites and their contribution to drug-drug interactions
Abstract
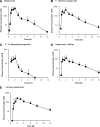
The aim of this study was to evaluate the contribution of metabolites to drug-drug interactions (DDI) using the inhibition of CYP2C19 and CYP3A4 by omeprazole and its metabolites as a model. Of the metabolites identified in vivo, 5-hydroxyomeprazole, 5'-O-desmethylomeprazole, omeprazole sulfone, and carboxyomeprazole had a metabolite to parent area under the plasma concentration-time curve (AUC(m)/AUC(p)) ratio ≥ 0.25 when either total or unbound concentrations were measured after a single 20-mg dose of omeprazole in a cocktail. All of the metabolites inhibited CYP2C19 and CYP3A4 reversibly. In addition omeprazole, omeprazole sulfone, and 5'-O-desmethylomeprazole were time dependent inhibitors (TDI) of CYP2C19, whereas omeprazole and 5'-O-desmethylomeprazole were found to be TDIs of CYP3A4. The in vitro inhibition constants and in vivo plasma concentrations were used to evaluate whether characterization of the metabolites affected DDI risk assessment. Identifying omeprazole as a TDI of both CYP2C19 and CYP3A4 was the most important factor in DDI risk assessment. Consideration of reversible inhibition by omeprazole and its metabolites would not identify DDI risk with CYP3A4, and with CYP2C19, reversible inhibition values would only identify DDI risk if the metabolites were included in the assessment. On the basis of inactivation data, CYP2C19 and CYP3A4 inhibition by omeprazole would be sufficient to identify risk, but metabolites were predicted to contribute 30-63% to the in vivo hepatic interactions. Therefore, consideration of metabolites may be important in quantitative predictions of in vivo DDIs. The results of this study show that, although metabolites contribute to in vivo DDIs, their relative abundance in circulation or logP values do not predict their contribution to in vivo DDI risk.
Figures



Similar articles
-
Stereoselective inhibition of CYP2C19 and CYP3A4 by fluoxetine and its metabolite: implications for risk assessment of multiple time-dependent inhibitor systems.Drug Metab Dispos. 2013 Dec;41(12):2056-65. doi: 10.1124/dmd.113.052639. Epub 2013 Jun 19. Drug Metab Dispos. 2013. PMID: 23785064 Free PMC article.
-
Herbal medicine yin zhi huang induces CYP3A4-mediated sulfoxidation and CYP2C19-dependent hydroxylation of omeprazole.Acta Pharmacol Sin. 2007 Oct;28(10):1685-92. doi: 10.1111/j.1745-7254.2007.00617.x. Acta Pharmacol Sin. 2007. PMID: 17883958 Clinical Trial.
-
Fluoxetine- and norfluoxetine-mediated complex drug-drug interactions: in vitro to in vivo correlation of effects on CYP2D6, CYP2C19, and CYP3A4.Clin Pharmacol Ther. 2014 Jun;95(6):653-62. doi: 10.1038/clpt.2014.50. Epub 2014 Feb 25. Clin Pharmacol Ther. 2014. PMID: 24569517 Free PMC article.
-
Clinical impact of CYP2C19 polymorphism on the action of proton pump inhibitors: a review of a special problem.Int J Clin Pharmacol Ther. 2006 Jul;44(7):297-302. doi: 10.5414/cpp44297. Int J Clin Pharmacol Ther. 2006. PMID: 16961157 Review.
-
Comparative pharmacokinetics of Omeprazole and its metabolites in poor and extensive metabolizer Pakistani healthy volunteers and a review of different studies.Pak J Pharm Sci. 2018 Jul;31(4):1363-1374. Pak J Pharm Sci. 2018. PMID: 30033421 Review.
Cited by
-
CYP2D6 Is Inducible by Endogenous and Exogenous Corticosteroids.Drug Metab Dispos. 2016 May;44(5):750-7. doi: 10.1124/dmd.115.069229. Epub 2016 Mar 10. Drug Metab Dispos. 2016. Retraction in: Drug Metab Dispos. 2018 Sep;46(9):1360. doi: 10.1124/dmd.115.069229retraction. PMID: 26965986 Free PMC article. Retracted.
-
Oral Drug Absorption and Drug Disposition in Critically Ill Cardiac Patients.Pharmaceutics. 2023 Nov 7;15(11):2598. doi: 10.3390/pharmaceutics15112598. Pharmaceutics. 2023. PMID: 38004576 Free PMC article.
-
Screening of cytochrome P450 3A4 inhibitors via in silico and in vitro approaches.RSC Adv. 2018 Oct 10;8(61):34783-34792. doi: 10.1039/c8ra06311g. eCollection 2018 Oct 10. RSC Adv. 2018. PMID: 35547066 Free PMC article.
-
The Influence of CYP3A4 Genetic Polymorphism and Proton Pump Inhibitors on Osimertinib Metabolism.Front Pharmacol. 2022 Mar 10;13:794931. doi: 10.3389/fphar.2022.794931. eCollection 2022. Front Pharmacol. 2022. PMID: 35359868 Free PMC article.
-
Mechanistic PBPK Modeling of Urine pH Effect on Renal and Systemic Disposition of Methamphetamine and Amphetamine.J Pharmacol Exp Ther. 2020 Jun;373(3):488-501. doi: 10.1124/jpet.120.264994. Epub 2020 Mar 20. J Pharmacol Exp Ther. 2020. PMID: 32198137 Free PMC article.
References
-
- Andersson T, Weidolf L. (2008) Stereoselective disposition of proton pump inhibitors. Clin Drug Investig 28:263–279 - PubMed
-
- Angiolillo DJ, Gibson CM, Cheng S, Ollier C, Nicolas O, Bergougnan L, Perrin L, LaCreta FP, Hurbin F, Dubar M. (2011) Differential effects of omeprazole and pantoprazole on the pharmacodynamics and pharmacokinetics of clopidogrel in healthy subjects: randomized, placebo-controlled, crossover comparison studies. Clin Pharmacol Ther 89:65–74 - PubMed
-
- Berry LM, Zhao Z. (2008) An examination of IC50 and IC50-shift experiments in assessing time-dependent inhibition of CYP3A4, CYP2D6 and CYP2C9 in human liver microsomes. Drug Metab Lett 2:51–59 - PubMed
-
- Boulenc X, Djebli N, Shi J, Perrin L, Brian W, Van Horn R, Hurbin F. (2012) Effects of omeprazole and genetic polymorphism of CYP2C19 on the clopidogrel active metabolite. Drug Metab Dispos 40:187–197 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

