Inhibition of CYP2C19 and CYP3A4 by omeprazole metabolites and their contribution to drug-drug interactions
- PMID: 23620487
- PMCID: PMC3684819
- DOI: 10.1124/dmd.113.051722
Inhibition of CYP2C19 and CYP3A4 by omeprazole metabolites and their contribution to drug-drug interactions
Abstract
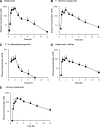
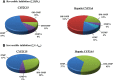
The aim of this study was to evaluate the contribution of metabolites to drug-drug interactions (DDI) using the inhibition of CYP2C19 and CYP3A4 by omeprazole and its metabolites as a model. Of the metabolites identified in vivo, 5-hydroxyomeprazole, 5'-O-desmethylomeprazole, omeprazole sulfone, and carboxyomeprazole had a metabolite to parent area under the plasma concentration-time curve (AUC(m)/AUC(p)) ratio ≥ 0.25 when either total or unbound concentrations were measured after a single 20-mg dose of omeprazole in a cocktail. All of the metabolites inhibited CYP2C19 and CYP3A4 reversibly. In addition omeprazole, omeprazole sulfone, and 5'-O-desmethylomeprazole were time dependent inhibitors (TDI) of CYP2C19, whereas omeprazole and 5'-O-desmethylomeprazole were found to be TDIs of CYP3A4. The in vitro inhibition constants and in vivo plasma concentrations were used to evaluate whether characterization of the metabolites affected DDI risk assessment. Identifying omeprazole as a TDI of both CYP2C19 and CYP3A4 was the most important factor in DDI risk assessment. Consideration of reversible inhibition by omeprazole and its metabolites would not identify DDI risk with CYP3A4, and with CYP2C19, reversible inhibition values would only identify DDI risk if the metabolites were included in the assessment. On the basis of inactivation data, CYP2C19 and CYP3A4 inhibition by omeprazole would be sufficient to identify risk, but metabolites were predicted to contribute 30-63% to the in vivo hepatic interactions. Therefore, consideration of metabolites may be important in quantitative predictions of in vivo DDIs. The results of this study show that, although metabolites contribute to in vivo DDIs, their relative abundance in circulation or logP values do not predict their contribution to in vivo DDI risk.
Figures



References
-
- Andersson T, Weidolf L. (2008) Stereoselective disposition of proton pump inhibitors. Clin Drug Investig 28:263–279 - PubMed
-
- Angiolillo DJ, Gibson CM, Cheng S, Ollier C, Nicolas O, Bergougnan L, Perrin L, LaCreta FP, Hurbin F, Dubar M. (2011) Differential effects of omeprazole and pantoprazole on the pharmacodynamics and pharmacokinetics of clopidogrel in healthy subjects: randomized, placebo-controlled, crossover comparison studies. Clin Pharmacol Ther 89:65–74 - PubMed
-
- Berry LM, Zhao Z. (2008) An examination of IC50 and IC50-shift experiments in assessing time-dependent inhibition of CYP3A4, CYP2D6 and CYP2C9 in human liver microsomes. Drug Metab Lett 2:51–59 - PubMed
-
- Boulenc X, Djebli N, Shi J, Perrin L, Brian W, Van Horn R, Hurbin F. (2012) Effects of omeprazole and genetic polymorphism of CYP2C19 on the clopidogrel active metabolite. Drug Metab Dispos 40:187–197 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

