Modulation of the endoplasmic reticulum-mitochondria interface in Alzheimer's disease and related models
- PMID: 23620518
- PMCID: PMC3651455
- DOI: 10.1073/pnas.1300677110
Modulation of the endoplasmic reticulum-mitochondria interface in Alzheimer's disease and related models
Abstract
It is well-established that subcompartments of endoplasmic reticulum (ER) are in physical contact with the mitochondria. These lipid raft-like regions of ER are referred to as mitochondria-associated ER membranes (MAMs), and they play an important role in, for example, lipid synthesis, calcium homeostasis, and apoptotic signaling. Perturbation of MAM function has previously been suggested in Alzheimer's disease (AD) as shown in fibroblasts from AD patients and a neuroblastoma cell line containing familial presenilin-2 AD mutation. The effect of AD pathogenesis on the ER-mitochondria interplay in the brain has so far remained unknown. Here, we studied ER-mitochondria contacts in human AD brain and related AD mouse and neuronal cell models. We found uniform distribution of MAM in neurons. Phosphofurin acidic cluster sorting protein-2 and σ1 receptor, two MAM-associated proteins, were shown to be essential for neuronal survival, because siRNA knockdown resulted in degeneration. Up-regulated MAM-associated proteins were found in the AD brain and amyloid precursor protein (APP)Swe/Lon mouse model, in which up-regulation was observed before the appearance of plaques. By studying an ER-mitochondria bridging complex, inositol-1,4,5-triphosphate receptor-voltage-dependent anion channel, we revealed that nanomolar concentrations of amyloid β-peptide increased inositol-1,4,5-triphosphate receptor and voltage-dependent anion channel protein expression and elevated the number of ER-mitochondria contact points and mitochondrial calcium concentrations. Our data suggest an important role of ER-mitochondria contacts and cross-talk in AD pathology.
Keywords: AD mouse models; hippocampal neurons; human cortical brain tissue.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

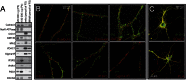

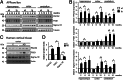
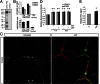

References
-
- Ankarcrona M, Mangialasche F, Winblad B. Rethinking Alzheimer’s disease therapy: Are mitochondria the key? J Alzheimers Dis. 2010;20(Suppl 2):S579–S590. - PubMed
-
- Rusiñol AE, Cui Z, Chen MH, Vance JE. A unique mitochondria-associated membrane fraction from rat liver has a high capacity for lipid synthesis and contains pre-Golgi secretory proteins including nascent lipoproteins. J Biol Chem. 1994;269(44):27494–27502. - PubMed
-
- Vance JE. Molecular and cell biology of phosphatidylserine and phosphatidylethanolamine metabolism. Prog Nucleic Acid Res Mol Biol. 2003;75(2003):69–111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

