Substrate-modulated cytochrome P450 17A1 and cytochrome b5 interactions revealed by NMR
- PMID: 23620596
- PMCID: PMC3675632
- DOI: 10.1074/jbc.M113.468926
Substrate-modulated cytochrome P450 17A1 and cytochrome b5 interactions revealed by NMR
Abstract
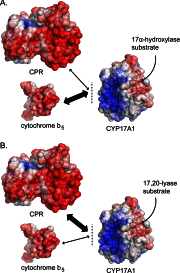
The membrane heme protein cytochrome b5 (b5) can enhance, inhibit, or have no effect on cytochrome P450 (P450) catalysis, depending on the specific P450, substrate, and reaction conditions, but the structural basis remains unclear. Here the interactions between the soluble domain of microsomal b5 and the catalytic domain of the bifunctional steroidogenic cytochrome P450 17A1 (CYP17A1) were investigated. CYP17A1 performs both steroid hydroxylation, which is unaffected by b5, and an androgen-forming lyase reaction that is facilitated 10-fold by b5. NMR chemical shift mapping of b5 titrations with CYP17A1 indicates that the interaction occurs in an intermediate exchange regime and identifies charged surface residues involved in the protein/protein interface. The role of these residues is confirmed by disruption of the complex upon mutagenesis of either the anionic b5 residues (Glu-48 or Glu-49) or the corresponding cationic CYP17A1 residues (Arg-347, Arg-358, or Arg-449). Cytochrome b5 binding to CYP17A1 is also mutually exclusive with binding of NADPH-cytochrome P450 reductase. To probe the differential effects of b5 on the two CYP17A1-mediated reactions and, thus, communication between the superficial b5 binding site and the buried CYP17A1 active site, CYP17A1/b5 complex formation was characterized with either hydroxylase or lyase substrates bound to CYP17A1. Significantly, the CYP17A1/b5 interaction is stronger when the hydroxylase substrate pregnenolone is present in the CYP17A1 active site than when the lyase substrate 17α-hydroxypregnenolone is in the active site. These findings form the basis for a clearer understanding of this important interaction by directly measuring the reversible binding of the two proteins, providing evidence of communication between the CYP17A1 active site and the superficial proximal b5 binding site.
Keywords: Cytochrome P450 17A1; Cytochrome b5; Enzyme Mechanisms; NMR; Protein Conformation; Protein-Protein Interactions; Steroidogenesis.
Figures




References
-
- Ortiz de Montellano P. R. (2005) Cytochrome P450. Structure, Mechanism, and Biochemistry, 3rd ed., pp. 133–134 and 448–450, Kluwer Academic/Plenum Publishers, New York
-
- Schenkman J. B., Jansson I. (2003) The many roles of cytochrome b5. Pharmacol. Ther. 97, 139–152 - PubMed
-
- Zhang H., Im S. C., Waskell L. (2007) Cytochrome b5 increases the rate of product formation by cytochrome P450 2B4 and competes with cytochrome P450 reductase for a binding site on cytochrome P450 2B4. J. Biol. Chem. 282, 29766–29776 - PubMed
-
- Akhtar M. K., Kelly S. L., Kaderbhai M. A. (2005) Cytochrome b(5) modulation of 17α-hydroxylase and 17–20 lyase (CYP17) activities in steroidogenesis. J. Endocrinol. 187, 267–274 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

