Simultaneous binding of the anti-cancer IgM monoclonal antibody PAT-SM6 to low density lipoproteins and GRP78
- PMID: 23620733
- PMCID: PMC3631193
- DOI: 10.1371/journal.pone.0061239
Simultaneous binding of the anti-cancer IgM monoclonal antibody PAT-SM6 to low density lipoproteins and GRP78
Abstract
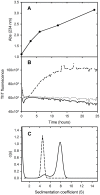
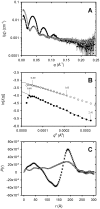
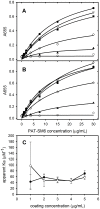
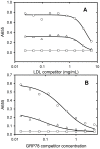
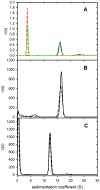
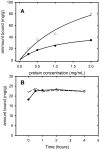
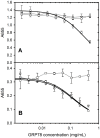
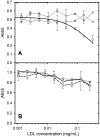
The tumour-derived monoclonal IgM antibody PAT-SM6 specifically kills malignant cells by an apoptotic mechanism linked to the excessive uptake of plasma lipids. The mechanism is postulated to occur via the multi-point attachment of PAT-SM6 to the unfolded protein response regulator GRP78, located on the surface of tumour cells, coupled to the simultaneous binding of plasma low density lipoprotein (LDL). We prepared and characterised LDL and oxidized LDL using sedimentation velocity and small-angle X-ray scattering (SAXS) analysis. Enzyme-linked immunosorbent (ELISA) techniques indicated apparent dissociation constants of approximately 20 nM for the binding of LDL or oxidized LDL to PAT-SM6. ELISA experiments showed cross competition with LDL inhibiting PAT-SM6 binding to immobilised GRP78, while, in the reverse experiment, GRP78 inhibited PAT-SM6 binding to immobilized LDL. In contrast to the results of the ELISA experiments, sedimentation velocity experiments indicated relatively weak interactions between LDL and PAT-SM6, suggesting immunoabsorbance to the microtiter plate is driven by an avidity-based binding mechanism. The importance of avidity and the multipoint attachment of antigens to PAT-SM6 was further investigated using antigen-coated polystyrene beads. Absorption of GRP78 or LDL to polystyrene microspheres led to an increase in the inhibition of PAT-SM6 binding to microtiter plates coated with GRP78 or LDL, respectively. These results support the hypothesis that the biological action of PAT-SM6 in tumour cell apoptosis depends on the multivalent nature of PAT-SM6 and the ability to interact simultaneously with LDL and multiple GRP78 molecules clustered on the tumour cell surface.
Conflict of interest statement
Figures
Similar articles
-
The anti-cancer IgM monoclonal antibody PAT-SM6 binds with high avidity to the unfolded protein response regulator GRP78.PLoS One. 2012;7(9):e44927. doi: 10.1371/journal.pone.0044927. Epub 2012 Sep 19. PLoS One. 2012. PMID: 23028685 Free PMC article.
-
Early development of PAT-SM6 for the treatment of melanoma.Melanoma Res. 2013 Aug;23(4):264-75. doi: 10.1097/CMR.0b013e328362cbc8. Melanoma Res. 2013. PMID: 23728394
-
The natural human IgM antibody PAT-SM6 induces apoptosis in primary human multiple myeloma cells by targeting heat shock protein GRP78.PLoS One. 2013 May 7;8(5):e63414. doi: 10.1371/journal.pone.0063414. Print 2013. PLoS One. 2013. PMID: 23667612 Free PMC article.
-
A GRP78-Directed Monoclonal Antibody Recaptures Response in Refractory Multiple Myeloma with Extramedullary Involvement.Clin Cancer Res. 2016 Sep 1;22(17):4341-9. doi: 10.1158/1078-0432.CCR-15-3111. Epub 2016 Mar 30. Clin Cancer Res. 2016. PMID: 27029491
-
GRP78-directed immunotherapy in relapsed or refractory multiple myeloma - results from a phase 1 trial with the monoclonal immunoglobulin M antibody PAT-SM6.Haematologica. 2015 Mar;100(3):377-84. doi: 10.3324/haematol.2014.117945. Epub 2015 Jan 30. Haematologica. 2015. PMID: 25637055 Free PMC article. Clinical Trial.
Cited by
-
Circulating IgM Requires Plasma Membrane Disruption to Bind Apoptotic and Non-Apoptotic Nucleated Cells and Erythrocytes.PLoS One. 2015 Jun 29;10(6):e0131849. doi: 10.1371/journal.pone.0131849. eCollection 2015. PLoS One. 2015. PMID: 26121639 Free PMC article.
References
-
- Pohle T, Brandlein S, Ruoff N, Muller-Hermelink HK, Vollmers HP (2004) Lipoptosis: tumor-specific cell death by antibody-induced intracellular lipid accumulation. Cancer Res 64: 3900–3906. - PubMed
-
- Brandlein S, Rauschert N, Rasche L, Dreykluft A, Hensel F, et al. (2007) The human IgM antibody SAM-6 induces tumor-specific apoptosis with oxidized low-density lipoprotein. Mol Cancer Ther 6: 326–333. - PubMed
-
- Rauschert N, Brandlein S, Holzinger E, Hensel F, Muller-Hermelink HK, et al. (2008) A new tumor-specific variant of GRP78 as target for antibody-based therapy. Lab Invest 88: 375–386. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

