Role of sensory experience in functional development of Drosophila motor circuits
- PMID: 23620812
- PMCID: PMC3631234
- DOI: 10.1371/journal.pone.0062199
Role of sensory experience in functional development of Drosophila motor circuits
Abstract
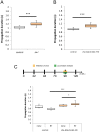
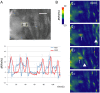
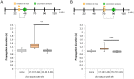
Neuronal circuits are formed according to a genetically predetermined program and then reconstructed in an experience-dependent manner. While the existence of experience-dependent plasticity has been demonstrated for the visual and other sensory systems, it remains unknown whether this is also the case for motor systems. Here we examined the effects of eliminating sensory inputs on the development of peristaltic movements in Drosophila embryos and larvae. The peristalsis is initially slow and uncoordinated, but gradually develops into a mature pattern during late embryonic stages. We tested whether inhibiting the transmission of specific sensory neurons during this period would have lasting effects on the properties of the sensorimotor circuits. We applied Shibire-mediated inhibition for six hours during embryonic development (15-21 h after egg laying [AEL]) and studied its effects on peristalsis in the mature second- and third-instar larvae. We found that inhibition of chordotonal organs, but not multidendritic neurons, led to a lasting decrease in the speed of larval locomotion. To narrow down the sensitive period, we applied shorter inhibition at various embryonic and larval stages and found that two-hour inhibition during 16-20 h AEL, but not at earlier or later stages, was sufficient to cause the effect. These results suggest that neural activity mediated by specific sensory neurons is involved in the maturation of sensorimotor circuits in Drosophila and that there is a critical period for this plastic change. Consistent with a role of chordotonal neurons in sensory feedback, these neurons were activated during larval peristalsis and acute inhibition of their activity decreased the speed of larval locomotion.
Conflict of interest statement
Figures



References
-
- Brown TG (1911) The intrinsic factors in the act of progression in the mammal. Proceedings of the Royal Society of London Series B-Containing Papers of a Biological Character 84: 308–319.
-
- Grillner S, Wallen P (1985) Central pattern generators for locomotion, with special reference to vertebrates. Annu Rev Neurosci 8: 233–261. - PubMed
-
- Buschges A (2005) Sensory control and organization of neural networks mediating coordination of multisegmental organs for locomotion. J Neurophysiol 93: 1127–1135. - PubMed
-
- Buonomano DV, Merzenich MM (1998) Cortical plasticity: from synapses to maps. Annu Rev Neurosci 21: 149–186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

