Neurotensin co-expressed in orexin-producing neurons in the lateral hypothalamus plays an important role in regulation of sleep/wakefulness states
- PMID: 23620827
- PMCID: PMC3631195
- DOI: 10.1371/journal.pone.0062391
Neurotensin co-expressed in orexin-producing neurons in the lateral hypothalamus plays an important role in regulation of sleep/wakefulness states
Abstract
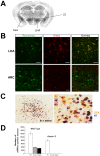
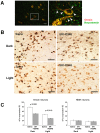
Both orexin and neurotensin are expressed in the lateral hypothalamic area (LHA) and have been implicated in the regulation of feeding, motor activity and the reward system. A double label immunofluorescence and in situ hybridization studies showed that neurotensin colocalizes with orexin in neurons of the LHA. Pharmacological studies suggested that neurotensin excites orexin-producing neurons (orexin neurons) through activation of neurotensin receptor-2 (NTSR-2) and non-selective cation channels. In situ hybridization study showed that most orexin neurons express neurotensin receptor-2 mRNA but not neurotensin receptor-1 (Ntsr-1) mRNA. Immunohistochemical studies showed that neurotensin-immunoreactive fibers make appositions to orexin neurons. A neurotensin receptor antagonist decreased Fos expression in orexin neurons and wakefulness time in wild type mice when administered intraperitoneally. However, the antagonist did not evoke any effect on these parameters in orexin neuron-ablated mice. These observations suggest the importance of neurotensin in maintaining activity of orexin neurons. The evidence presented here expands our understanding of the regulatory mechanism of orexin neurons.
Conflict of interest statement
Figures


References
-
- Dicou E, Vincent JP, Mazella J (2004) Neurotensin receptor-3/sortilin mediates neurotensin-induced cytokine/chemokine expression in a murine microglial cell line. J Neurosci Res 78: 92–99. - PubMed
-
- Chabry J, Labbe-Jullie C, Gully D, Kitabgi P, Vincent JP, et al. (1994) Stable expression of the cloned rat brain neurotensin receptor into fibroblasts: binding properties, photoaffinity labeling, transduction mechanisms, and internalization. J Neurochem 63: 19–27. - PubMed
-
- Najimi M, Gailly P, Maloteaux JM, Hermans E (2002) Distinct regions of C-terminus of the high affinity neurotensin receptor mediate the functional coupling with pertussis toxin sensitive and insensitive G-proteins. FEBS Lett 512: 329–333. - PubMed
-
- Chalon P, Vita N, Kaghad M, Guillemot M, Bonnin J, et al. (1996) Molecular cloning of a levocabastine-sensitive neurotensin binding site. FEBS Lett 386: 91–94. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

