Hypofractionated three-dimensional conformal radiotherapy for medically inoperable early stage non-small-cell lung cancer
- PMID: 23620865
- PMCID: PMC3633227
- DOI: 10.3857/roj.2013.31.1.18
Hypofractionated three-dimensional conformal radiotherapy for medically inoperable early stage non-small-cell lung cancer
Abstract
Purpose: The purpose of this study was to assess the clinical outcomes of hypofractionated radiotherapy (HFRT) with three-dimensional conformal technique for medically inoperable patients with early stage non-small-cell lung cancer (NSCLC) and to evaluate prognostic factors.
Materials and methods: We performed a retrospective review of 26 patients who underwent HFRT for early stage NSCLC between September 2005 and August 2011. Only clinical stage T1-3N0 was included. The median RT dose was 70 Gy (range, 60 to 72 Gy) and the median biologically equivalent dose (BED) was 94.5 Gy (range, 78.0 to 100.8 Gy). In 84.6% of patients, 4 Gy per fraction was used. Neoadjuvant chemotherapy with paclitaxel and cisplatin was given to 2 of 26 patients.
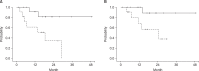
Results: The median follow-up time for surviving patients was 21 months (range, 13 to 49 months). The overall response rate was 53.9%, and the initial local control rate was 100%. The median survival duration was 27.8 months. Rates of 2-year overall survival, progression-free survival (PFS), local control (LC), and locoregional-free survival (LRFS) were 54.3%, 61.1%, 74.6%, and 61.9%, respectively. Multivariate analysis showed that BED (>90 vs. ≤90 Gy) was an independent prognostic factor influencing PFS, LC, and LRFS. Severe toxicities over grade 3 were not observed.
Conclusion: Radical HFRT can yield satisfactory disease control with acceptable rates of toxicities in medically inoperable patients with early stage NSCLC. HFRT is a viable alternative for clinics and patients ineligible for stereotactic ablative radiotherapy. BED over 90 Gy and 4 Gy per fraction might be appropriate for HFRT.
Keywords: Early stage; Hypofractionated radiotherapy; Medically inoperable; Non-small-cell lung cancer.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures
References
-
- Scott WJ, Howington J, Feigenberg S, Movsas B, Pisters K American College of Chest Physicians. Treatment of non-small cell lung cancer stage I and stage II: ACCP evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(3 Suppl):234S–242S. - PubMed
-
- Kaskowitz L, Graham MV, Emami B, Halverson KJ, Rush C. Radiation therapy alone for stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 1993;27:517–523. - PubMed
-
- Qiao X, Tullgren O, Lax I, Sirzen F, Lewensohn R. The role of radiotherapy in treatment of stage I non-small cell lung cancer. Lung Cancer. 2003;41:1–11. - PubMed
-
- Fowler JF, Chappell R. Non-small cell lung tumors repopulate rapidly during radiation therapy. Int J Radiat Oncol Biol Phys. 2000;46:516–517. - PubMed
-
- Bradley J, Graham MV, Winter K, et al. Toxicity and outcome results of RTOG 9311: a phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:318–328. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources