Remodeling of dendrites and spines in the C1q knockout model of genetic epilepsy
- PMID: 23621154
- PMCID: PMC3700654
- DOI: 10.1111/epi.12195
Remodeling of dendrites and spines in the C1q knockout model of genetic epilepsy
Abstract
Purpose: To determine whether developmental synaptic pruning defects in epileptic C1q-knockout (KO) mice are accompanied by postsynaptic abnormalities in dendrites and/or spines.
Methods: Immunofluorescence staining was performed on biocytin-filled layer Vb pyramidal neurons in sensorimotor cortex. Basal dendritic arbors and their spines were reconstructed with NEUROLUCIDA software, and their morphologic characteristics were quantitated in Neuroexplorer.
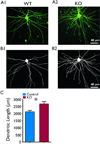
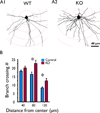
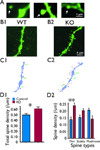
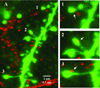
Key findings: Seven to nine completely filled pyramidal neurons were analyzed from the wild-type (WT) and C1q KO groups. Compared to WT controls, KO mice showed significant structural modifications in their basal dendrites including (1) higher density of dendritic spines (0.60 ± 0.03/μm vs. 0.49 ± 0.03/μm dendritic length in WT, p < 0.05); (2) remarkably increased occurrence of thin spines (0.26 ± 0.02/μm vs. 0.14 ± 0.02/μm dendritic length in control, p < 0.01); (3) longer dendritic length (2,680 ± 159 μm vs. 2,119 ± 108 μm in control); and (4) increased branching (22.6 ± 1.9 vs. 16.2 ± 1.3 in WT at 80 μm from soma center, p < 0.05; 12.4 ± 1.4 vs. 8.2 ± 0.6 in WT at 120 μm from soma center, respectively, p < 0.05). Dual immunolabeling demonstrated the expression of putative glutamate receptor 2 (GluR2) on some thin spines. These dendritic alterations are likely postsynaptic structural consequences of failure of synaptic pruning in the C1q KO mice.
Significance: Failure to prune excessive excitatory synapses in C1q KO mice is a likely mechanism underlying abnormalities in postsynaptic dendrites, including increased branching and alterations in spine type and density. It is also possible that seizure activity contributes to these abnormalities. These structural abnormalities, together with increased numbers of excitatory synapses, likely contribute to epileptogenesis in C1q KO mice.
Keywords: C1q; Dendrite; Genetic epilepsy; Pruning; Spine.
Wiley Periodicals, Inc. © 2013 International League Against Epilepsy.
Conflict of interest statement
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures
Similar articles
-
Enhanced synaptic connectivity and epilepsy in C1q knockout mice.Proc Natl Acad Sci U S A. 2010 Apr 27;107(17):7975-80. doi: 10.1073/pnas.0913449107. Epub 2010 Apr 7. Proc Natl Acad Sci U S A. 2010. PMID: 20375278 Free PMC article.
-
Evaluation of dendrite morphology in Wistar and genetic absence epileptic rats.Brain Struct Funct. 2024 Dec 16;230(1):5. doi: 10.1007/s00429-024-02868-3. Brain Struct Funct. 2024. PMID: 39681662
-
The glutamate receptor 2 subunit controls post-synaptic density complexity and spine shape in the dentate gyrus.Eur J Neurosci. 2008 Jan;27(2):315-25. doi: 10.1111/j.1460-9568.2007.06005.x. Eur J Neurosci. 2008. PMID: 18215230
-
Spine loss and other dendritic abnormalities in epilepsy.Hippocampus. 2000;10(5):617-25. doi: 10.1002/1098-1063(2000)10:5<617::AID-HIPO13>3.0.CO;2-R. Hippocampus. 2000. PMID: 11075833 Review.
-
Dendritic spine pathology in epilepsy: cause or consequence?Neuroscience. 2013 Oct 22;251:141-50. doi: 10.1016/j.neuroscience.2012.03.048. Epub 2012 Apr 20. Neuroscience. 2013. PMID: 22522469 Review.
Cited by
-
Microglia facilitate and stabilize the response to general anesthesia via modulating the neuronal network in a brain region-specific manner.Elife. 2023 Dec 22;12:RP92252. doi: 10.7554/eLife.92252. Elife. 2023. PMID: 38131301 Free PMC article.
-
The endogenous neuronal complement inhibitor SRPX2 protects against complement-mediated synapse elimination during development.Nat Neurosci. 2020 Sep;23(9):1067-1078. doi: 10.1038/s41593-020-0672-0. Epub 2020 Jul 13. Nat Neurosci. 2020. PMID: 32661396 Free PMC article.
-
C1q as a target molecule to treat human disease: What do mouse studies teach us?Front Immunol. 2022 Aug 3;13:958273. doi: 10.3389/fimmu.2022.958273. eCollection 2022. Front Immunol. 2022. PMID: 35990646 Free PMC article.
-
Rapid Golgi analysis method for efficient and unbiased classification of dendritic spines.PLoS One. 2014 Sep 10;9(9):e107591. doi: 10.1371/journal.pone.0107591. eCollection 2014. PLoS One. 2014. PMID: 25208214 Free PMC article.
-
Complement System in Alzheimer's Disease.Int J Mol Sci. 2021 Dec 20;22(24):13647. doi: 10.3390/ijms222413647. Int J Mol Sci. 2021. PMID: 34948444 Free PMC article. Review.
References
-
- Aliashkevich AF, Yilmazer-Hanke D, Van Roost D, Mundhenk B, Schramm J, Blumcke I. Cellular pathology of amygdala neurons in human temporal lobe epilepsy. Acta Neuropathol. 2003;106:99–106. - PubMed
-
- Ampuero E, Dagnino-Subiabre A, Sandoval R, Zepeda-Carreno R, Sandoval S, Viedma A, Aboitiz F, Orrego F, Wyneken U. Status epilepticus induces region-specific changes in dendritic spines, dendritic length and TrkB protein content of rat brain cortex. Brain Res. 2007;1150:225–238. - PubMed
-
- Ballesteros-Yanez I, Benavides-Piccione R, Elston GN, Yuste R, Defelipe J. Density and morphology of dendritic spines in mouse neocortex. Neuroscience. 2006;138:403–409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

