Parenteral ascorbate as a cancer therapeutic: a reassessment based on pharmacokinetics
- PMID: 23621620
- PMCID: PMC3869468
- DOI: 10.1089/ars.2013.5372
Parenteral ascorbate as a cancer therapeutic: a reassessment based on pharmacokinetics
Abstract
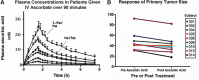
Significance: Ewan Cameron reported that ascorbate, given orally and intravenously at doses of up to 10 g/day, was effective in the treatment of cancer. Double-blind placebo-controlled clinical trials showed no survival advantage when the same doses of ascorbate were given orally, leading the medical and scientific communities to dismiss the use of ascorbate as a potential cancer treatment. However, the route of administration results in major differences in ascorbate bioavailability. Tissue and plasma concentrations are tightly controlled in response to oral administration, but this can be bypassed by intravenous administration. These data provide a plausible scientific rationale for the absence of a response to orally administered ascorbate in the Mayo clinic trials and indicate the need to reassess ascorbate as a cancer therapeutic.
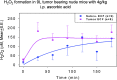
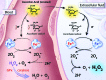
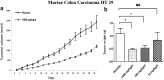
Recent advances: High dose ascorbate is selectively cytotoxic to cancer cell lines through the generation of extracellular hydrogen peroxide (H2O2). Murine xenograft models confirm a growth inhibitory effect of pharmacological concentrations. The safety of intravenous ascorbate has been verified in encouraging pilot clinical studies.
Critical issues: Neither the selective toxicity of pharmacologic ascorbate against cancer cells nor the mechanism of H2O2-mediated cytotoxicity is fully understood. Despite promising preclinical data, the question of clinical efficacy remains.
Future directions: A full delineation of mechanism is of interest because it may indicate susceptible cancer types. Effects of pharmacologic ascorbate used in combination with standard treatments need to be defined. Most importantly, the clinical efficacy of ascorbate needs to be reassessed using proper dosing, route of administration, and controls.
Figures



References
-
- Abdel-Latif MM, Raouf AA, Sabra K, Kelleher D, and Reynolds JV. Vitamin C enhances chemosensitization of esophageal cancer cells in vitro. J Chemother 17: 539–549, 2005 - PubMed
-
- Andersson DA, Gentry C, Alenmyr L, Killander D, Lewis SE, Andersson A, Bucher B, Galzi JL, Sterner O, Bevan S, Hogestatt ED, and Zygmunt PM. TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Delta(9)-tetrahydrocannabiorcol. Nat Commun 2: 551, 2011 - PubMed
-
- Bown SR. Scurvy: How a Surgeon, a Mariner, and a Gentleman Solved the Greatest Medical Mystery of the Age of Sail. New York: Thomas Dunne Books, 2004, p 254
-
- Cameron E. and Campbell A. The orthomolecular treatment of cancer. II. Clinical trial of high-dose ascorbic acid supplements in advanced human cancer. Chem Biol Interact 9: 285–315, 1974 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

