MMP-2 mediates local degradation and remodeling of collagen by annulus fibrosus cells of the intervertebral disc
- PMID: 23621950
- PMCID: PMC4060574
- DOI: 10.1186/ar4224
MMP-2 mediates local degradation and remodeling of collagen by annulus fibrosus cells of the intervertebral disc
Abstract
Introduction: Degeneration of the intervertebral disc (IVD) is characterized by marked degradation and restructuring of the annulus fibrosus (AF). Although several matrix metalloproteinases (MMPs) have been found to be more prevalent in degenerate discs, their coordination and function within the context of the disease process are still not well understood. In this study, we sought to determine whether MMP-2 is associated with degenerative changes in the AF and to identify the manner by which AF cells use MMP-2.
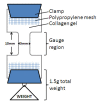
Methods: Two established animal models of disc degeneration, static compression and transannular needle puncture of rodent caudal discs, were examined for MMP-2 immunopositivity. With lentiviral transduction of an shRNA expression cassette, we screened and identified an effective shRNA sequence for generating stable RNA interference to silence MMP-2 expression in primary rat AF cells. Gelatin films were used to compare gelatinase activity and spatial patterns of degradation between transduced cells, and both noninfected and nonsense shRNA controls. The functional significance of MMP-2 was determined by assessing the ability for cells to remodel collagen gels.
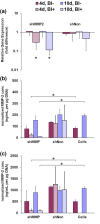
Results: Both static compression and 18-g annular puncture of rodent caudal discs stimulated an increase in MMP-2 activity with concurrent lamellar disorganization in the AF, whereas 22-g and 26-g needle injuries did not. To investigate the functional role of MMP-2, we established lentivirus-mediated RNAi to induce stable knockdown of transcript levels by as much as 88%, and protein levels by as much as 95% over a 10-day period. Culturing transduced cells on gelatin films confirmed that MMP-2 is the primary functional gelatinase in AF cells, and that MMP-2 is used locally in regions immediately around AF cells. In collagen gels, transduced cells demonstrated an inability to remodel collagen matrices.
Conclusions: Our study indicates that increases in MMP-2 observed in human degenerate discs are mirrored in experimentally induced degenerative changes in rodent animal models. AF cells appear to use MMP-2 in a very directed fashion for local matrix degradation and collagen remodeling. This suggests that MMP-2 may have a functionally significant role in the etiology of degenerative disc disease and could be a potential therapeutic target.
Figures




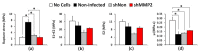
Similar articles
-
Rat tail static compression model mimics extracellular matrix metabolic imbalances of matrix metalloproteinases, aggrecanases, and tissue inhibitors of metalloproteinases in intervertebral disc degeneration.Arthritis Res Ther. 2012 Mar 6;14(2):R51. doi: 10.1186/ar3764. Arthritis Res Ther. 2012. PMID: 22394620 Free PMC article.
-
Low energy extracorporeal shock wave therapy combined with low tension traction can better reshape the microenvironment in degenerated intervertebral disc regeneration and repair.Spine J. 2021 Jan;21(1):160-177. doi: 10.1016/j.spinee.2020.08.004. Epub 2020 Aug 13. Spine J. 2021. PMID: 32800896
-
Mechanical destabilization induced by controlled annular incision of the intervertebral disc dysregulates metalloproteinase expression and induces disc degeneration.Spine (Phila Pa 1976). 2012 Jan 1;37(1):18-25. doi: 10.1097/BRS.0b013e31820cd8d5. Spine (Phila Pa 1976). 2012. PMID: 22179320
-
Effect of Static Compression Loads on Intervertebral Disc: An in Vivo Bent Rat Tail Model.Orthop Surg. 2018 May;10(2):134-143. doi: 10.1111/os.12377. Epub 2018 May 16. Orthop Surg. 2018. PMID: 29770581 Free PMC article.
-
Total disc replacement using a tissue-engineered intervertebral disc in vivo: new animal model and initial results.Evid Based Spine Care J. 2010 Aug;1(2):62-6. doi: 10.1055/s-0028-1100918. Evid Based Spine Care J. 2010. PMID: 23637671 Free PMC article.
Cited by
-
USP14 promotes pyroptosis of human annulus fibrosus cells derived from patients with intervertebral disc degeneration through deubiquitination of NLRP3.Acta Biochim Biophys Sin (Shanghai). 2022 Nov 25;54(11):1720 - 1730. doi: 10.3724/abbs.2022171. Acta Biochim Biophys Sin (Shanghai). 2022. PMID: 36514221 Free PMC article.
-
The lysosomal trafficking regulator is necessary for normal wound healing.Wound Repair Regen. 2022 Jan;30(1):82-99. doi: 10.1111/wrr.12984. Epub 2021 Nov 27. Wound Repair Regen. 2022. PMID: 34837653 Free PMC article.
-
Tissue-specific parameters for the design of ECM-mimetic biomaterials.Acta Biomater. 2021 Sep 15;132:83-102. doi: 10.1016/j.actbio.2021.04.017. Epub 2021 Apr 18. Acta Biomater. 2021. PMID: 33878474 Free PMC article. Review.
-
Immunobiology of periprosthetic inflammation and pain following ultra-high-molecular-weight-polyethylene wear debris in the lumbar spine.Expert Rev Clin Immunol. 2018 Aug;14(8):695-706. doi: 10.1080/1744666X.2018.1511428. Epub 2018 Aug 21. Expert Rev Clin Immunol. 2018. PMID: 30099915 Free PMC article. Review.
-
Matrix Metallopeptidase-2 Gene rs2287074 Polymorphism is Associated with Brick Tea Skeletal Fluorosis in Tibetans and Kazaks, China.Sci Rep. 2017 Jan 12;7:40086. doi: 10.1038/srep40086. Sci Rep. 2017. PMID: 28079131 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

