Evidence for tankyrases as antineoplastic targets in lung cancer
- PMID: 23621985
- PMCID: PMC3644501
- DOI: 10.1186/1471-2407-13-211
Evidence for tankyrases as antineoplastic targets in lung cancer
Abstract
Background: New pharmacologic targets are urgently needed to treat or prevent lung cancer, the most common cause of cancer death for men and women. This study identified one such target. This is the canonical Wnt signaling pathway, which is deregulated in cancers, including those lacking adenomatous polyposis coli or β-catenin mutations. Two poly-ADP-ribose polymerase (PARP) enzymes regulate canonical Wnt activity: tankyrase (TNKS) 1 and TNKS2. These enzymes poly-ADP-ribosylate (PARsylate) and destabilize axin, a key component of the β-catenin phosphorylation complex.
Methods: This study used comprehensive gene profiles to uncover deregulation of the Wnt pathway in murine transgenic and human lung cancers, relative to normal lung. Antineoplastic consequences of genetic and pharmacologic targeting of TNKS in murine and human lung cancer cell lines were explored, and validated in vivo in mice by implantation of murine transgenic lung cancer cells engineered with reduced TNKS expression relative to controls.
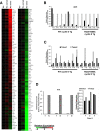
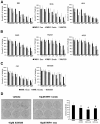
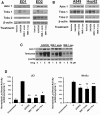
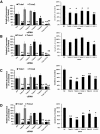
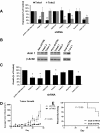
Results: Microarray analyses comparing Wnt pathway members in malignant versus normal tissues of a murine transgenic cyclin E lung cancer model revealed deregulation of Wnt pathway components, including TNKS1 and TNKS2. Real-time PCR assays independently confirmed these results in paired normal-malignant murine and human lung tissues. Individual treatments of a panel of human and murine lung cancer cell lines with the TNKS inhibitors XAV939 and IWR-1 dose-dependently repressed cell growth and increased cellular axin 1 and tankyrase levels. These inhibitors also repressed expression of a Wnt-responsive luciferase construct, implicating the Wnt pathway in conferring these antineoplastic effects. Individual or combined knockdown of TNKS1 and TNKS2 with siRNAs or shRNAs reduced lung cancer cell growth, stabilized axin, and repressed tumor formation in murine xenograft and syngeneic lung cancer models.
Conclusions: Findings reported here uncovered deregulation of specific components of the Wnt pathway in both human and murine lung cancer models. Repressing TNKS activity through either genetic or pharmacological approaches antagonized canonical Wnt signaling, reduced murine and human lung cancer cell line growth, and decreased tumor formation in mouse models. Taken together, these findings implicate the use of TNKS inhibitors to target the Wnt pathway to combat lung cancer.
Figures

References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. - PubMed
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Moolgavkar SH, Holford TR, Levy DT, Kong CY, Foy M, Clarke L, Jeon J, Hazelton WD, Meza R, Schultz F, McCarthy W, Boer R, Gorlova O, Gazelle GS, Kimmel M, McMahon PM, De Koning HJ, Feuer EJ. Impact of reduced tobacco smoking on lung cancer mortality in the united states during 1975–2000. J Natl Cancer Inst. 2012;104:541–548. - PMC - PubMed
-
- Yang P. Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest. 2005;128:452–462. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

