Parity induces differentiation and reduces Wnt/Notch signaling ratio and proliferation potential of basal stem/progenitor cells isolated from mouse mammary epithelium
- PMID: 23621987
- PMCID: PMC3672662
- DOI: 10.1186/bcr3419
Parity induces differentiation and reduces Wnt/Notch signaling ratio and proliferation potential of basal stem/progenitor cells isolated from mouse mammary epithelium
Abstract
Introduction: Early pregnancy has a strong protective effect against breast cancer in humans and rodents, but the underlying mechanism is unknown. Because breast cancers are thought to arise from specific cell subpopulations of mammary epithelia, we studied the effect of parity on the transcriptome and the differentiation/proliferation potential of specific luminal and basal mammary cells in mice.
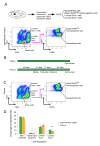
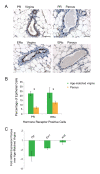
Methods: Mammary epithelial cell subpopulations (luminal Sca1-, luminal Sca1+, basal stem/progenitor, and basal myoepithelial cells) were isolated by flow cytometry from parous and age-matched virgin mice and examined by using a combination of unbiased genomics, bioinformatics, in vitro colony formation, and in vivo limiting dilution transplantation assays. Specific findings were further investigated with immunohistochemistry in entire glands of parous and age-matched virgin mice.
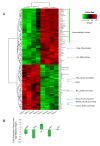
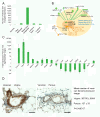
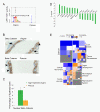
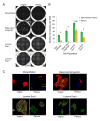
Results: Transcriptome analysis revealed an upregulation of differentiation genes and a marked decrease in the Wnt/Notch signaling ratio in basal stem/progenitor cells of parous mice. Separate bioinformatics analyses showed reduced activity for the canonical Wnt transcription factor LEF1/TCF7 and increased activity for the Wnt repressor TCF3. This finding was specific for basal stem/progenitor cells and was associated with downregulation of potentially carcinogenic pathways and a reduction in the proliferation potential of this cell subpopulation in vitro and in vivo. As a possible mechanism for decreased Wnt signaling in basal stem/progenitor cells, we found a more than threefold reduction in the expression of the secreted Wnt ligand Wnt4 in total mammary cells from parous mice, which corresponded to a similar decrease in the proportion of Wnt4-secreting and estrogen/progesterone receptor-positive cells. Because recombinant Wnt4 rescued the proliferation defect of basal stem/progenitor cells in vitro, reduced Wnt4 secretion appears to be causally related to parity-induced alterations of basal stem/progenitor cell properties in mice.
Conclusions: By revealing that parity induces differentiation and downregulates the Wnt/Notch signaling ratio and the in vitro and in vivo proliferation potential of basal stem/progenitor cells in mice, our study sheds light on the long-term consequences of an early pregnancy. Furthermore, it opens the door to future studies assessing whether inhibitors of the Wnt pathway may be used to mimic the parity-induced protective effect against breast cancer.
Figures

References
-
- Thordarson G, Jin E, Guzman RC, Swanson SM, Nandi S, Talamantes F. Refractoriness to mammary tumorigenesis in parous rats: is it caused by persistent changes in the hormonal environment or permanent biochemical alterations in the mammary epithelia? Carcinogenesis. 1995;16:2847–2853. doi: 10.1093/carcin/16.11.2847. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

