Development of dextran nanoparticles for stabilizing delicate proteins
- PMID: 23622054
- PMCID: PMC3655889
- DOI: 10.1186/1556-276X-8-197
Development of dextran nanoparticles for stabilizing delicate proteins
Abstract
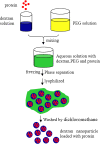
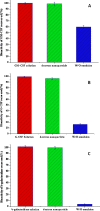
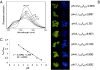
One of the most challenging problems in the development of protein pharmaceuticals is to deal with stabilities of proteins due to its complicated structures. This study aims to develop a novel approach to stabilize and encapsulate proteins into dextran nanoparticles without contacting the interface between the aqueous phase and the organic phase. The bovine serum albumin, granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), β-galactosidase, and myoglobin were selected as model proteins. The proteins were added into an aqueous solution containing the dextran and polyethylene glycol, and then encapsulated into dextran nanoparticles by aqueous-aqueous freezing-induced phase separation. The encapsulation efficiency and recovery of dextran nanoparticles were determined. The dextran nanoparticles loaded with proteins were characterized by scanning electron microscopy and particle size analysis. The protein aggregation was determined by size-exclusion chromatography-high-performance chromatography, and the bioactivity of proteins recovered during formulation steps was determined. The bioactivity of GM-CSF, G-CSF, and β-galactosidase were examined by the proliferation of TF-1 cell, NSF-60 cell, and ortho-nitrophenyl-β-galactoside assay, respectively. The results of bioactivity recovered show that this novel dextran nanoparticle can preserve the protein's bioactivity during the preparation process. LysoSensor™ Yellow/Blue dextran, a pH-sensitive indicator with fluorescence excited at two channels, was encapsulated into dextran nanoparticles to investigate the ability of dextran nanoparticles to resist the acidic microenvironment (pH < 2.5). The result shows that the dextran nanoparticles attenuate the acidic microenvironment in the poly (lactic-co-glycolic acid) microsphere by means of the dilution effect. These novel dextran nanoparticles provided an appealing approach to stabilize the delicate proteins for administration.
Figures




Similar articles
-
Formulation of sustained-release microspheres of granulocyte macrophage colony stimulating factor by freezing-induced phase separation with dextran and encapsulation with blended polymers.J Microencapsul. 2011;28(8):743-51. doi: 10.3109/02652048.2011.615950. Epub 2011 Oct 3. J Microencapsul. 2011. PMID: 21967463
-
Preparation of protein-loaded sustained-release microspheres via 'solid-in-oil-in-hydrophilic oil-in-ethanol (S/O/hO/E)' emulsification.Colloids Surf B Biointerfaces. 2010 Sep 1;79(2):326-33. doi: 10.1016/j.colsurfb.2010.04.004. Epub 2010 Apr 24. Colloids Surf B Biointerfaces. 2010. PMID: 20483570
-
Formulation and In Vitro Characterization of PLGA/PLGA-PEG Nanoparticles Loaded with Murine Granulocyte-Macrophage Colony-Stimulating Factor.AAPS PharmSciTech. 2021 Jun 24;22(5):191. doi: 10.1208/s12249-021-02049-z. AAPS PharmSciTech. 2021. PMID: 34169366 Free PMC article.
-
Sustained-release G-CSF microspheres using a novel solid-in-oil-in-oil-in-water emulsion method.Int J Nanomedicine. 2012;7:4559-69. doi: 10.2147/IJN.S33993. Epub 2012 Aug 17. Int J Nanomedicine. 2012. PMID: 22923993 Free PMC article.
-
Practically feasible production of sustained-release microspheres of granulocyte-macrophage colony-stimulating factor (rhGM-CSF).J Control Release. 2017 Aug 10;259:195-202. doi: 10.1016/j.jconrel.2017.04.004. Epub 2017 Apr 5. J Control Release. 2017. PMID: 28389408
Cited by
-
A Comprehensive Review of Nanoparticles: From Classification to Application and Toxicity.Molecules. 2024 Jul 25;29(15):3482. doi: 10.3390/molecules29153482. Molecules. 2024. PMID: 39124888 Free PMC article. Review.
-
Advances of magnetic nanoparticles in environmental application: environmental remediation and (bio)sensors as case studies.Environ Sci Pollut Res Int. 2018 Nov;25(31):30863-30879. doi: 10.1007/s11356-018-3095-7. Epub 2018 Sep 8. Environ Sci Pollut Res Int. 2018. PMID: 30196461 Review.
-
Promoting early neovascularization of SIS-repaired abdominal wall by controlled release of bioactive VEGF.RSC Adv. 2018 Jan 25;8(9):4548-4560. doi: 10.1039/c7ra11954b. eCollection 2018 Jan 24. RSC Adv. 2018. PMID: 35539528 Free PMC article.
-
Dextran Formulations as Effective Delivery Systems of Therapeutic Agents.Molecules. 2023 Jan 21;28(3):1086. doi: 10.3390/molecules28031086. Molecules. 2023. PMID: 36770753 Free PMC article. Review.
-
Amphiphilic dextran-vinyl laurate-based nanoparticles: formation, characterization, encapsulation, and cytotoxicity on human intestinal cell line.Prog Biomater. 2020 Jun;9(1-2):15-23. doi: 10.1007/s40204-020-00128-1. Epub 2020 Feb 18. Prog Biomater. 2020. PMID: 32072566 Free PMC article.
References
-
- Hermeling S, Crommelin DJS, Schellekens H, Jiskoot W. Development of a transgenic mouse model immune tolerant for human interferon beta. Adv Drug Deliver Rev. 2004;8:847–851. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

