Control of cell identity in pancreas development and regeneration
- PMID: 23622126
- PMCID: PMC3639438
- DOI: 10.1053/j.gastro.2013.01.074
Control of cell identity in pancreas development and regeneration
Abstract
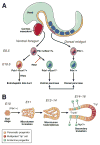
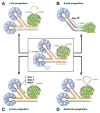
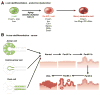
The endocrine and exocrine cells in the adult pancreas are not static, but can change their differentiation state in response to injury or stress. This concept of cells in flux means that there may be ways to generate certain types of cells (such as insulin-producing β-cells) and prevent formation of others (such as transformed neoplastic cells). We review different aspects of cell identity in the pancreas, discussing how cells achieve their identity during embryonic development and maturation, and how this identity remains plastic, even in the adult pancreas.
Copyright © 2013 AGA Institute. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Pavlov I. The Work of the Digestive Glands. Charles Griffin; 1902.
-
- Heller RS. The comparative anatomy of islets. Adv Exp Med Biol. 2010;654:21–37. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

