ADAR1 forms a complex with Dicer to promote microRNA processing and RNA-induced gene silencing
- PMID: 23622242
- PMCID: PMC3651894
- DOI: 10.1016/j.cell.2013.03.024
ADAR1 forms a complex with Dicer to promote microRNA processing and RNA-induced gene silencing
Abstract
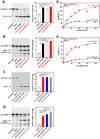
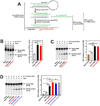
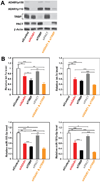
Adenosine deaminases acting on RNA (ADARs) are involved in RNA editing that converts adenosine residues to inosine specifically in double-stranded RNAs. In this study, we investigated the interaction of the RNA editing mechanism with the RNA interference (RNAi) machinery and found that ADAR1 forms a complex with Dicer through direct protein-protein interaction. Most importantly, ADAR1 increases the maximum rate (Vmax) of pre-microRNA (miRNA) cleavage by Dicer and facilitates loading of miRNA onto RNA-induced silencing complexes, identifying a new role of ADAR1 in miRNA processing and RNAi mechanisms. ADAR1 differentiates its functions in RNA editing and RNAi by the formation of either ADAR1/ADAR1 homodimer or Dicer/ADAR1 heterodimer complexes, respectively. As expected, the expression of miRNAs is globally inhibited in ADAR1(-/-) mouse embryos, which, in turn, alters the expression of their target genes and might contribute to their embryonic lethal phenotype.
Copyright © 2013 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Antagonistic and stimulative roles of ADAR1 in RNA silencing.RNA Biol. 2013 Aug;10(8):1240-7. doi: 10.4161/rna.25947. Epub 2013 Jul 30. RNA Biol. 2013. PMID: 23949595 Free PMC article.
-
Processing of Alu small RNAs by DICER/ADAR1 complexes and their RNAi targets.RNA. 2020 Dec;26(12):1801-1814. doi: 10.1261/rna.076745.120. Epub 2020 Aug 17. RNA. 2020. PMID: 32817447 Free PMC article.
-
MicroRNA-mediated loss of ADAR1 in metastatic melanoma promotes tumor growth.J Clin Invest. 2013 Jun;123(6):2703-18. doi: 10.1172/JCI62980. J Clin Invest. 2013. PMID: 23728176 Free PMC article.
-
A-to-I editing of coding and non-coding RNAs by ADARs.Nat Rev Mol Cell Biol. 2016 Feb;17(2):83-96. doi: 10.1038/nrm.2015.4. Epub 2015 Dec 9. Nat Rev Mol Cell Biol. 2016. PMID: 26648264 Free PMC article. Review.
-
Adenosine deaminases acting on RNA, RNA editing, and interferon action.J Interferon Cytokine Res. 2011 Jan;31(1):99-117. doi: 10.1089/jir.2010.0097. Epub 2010 Dec 23. J Interferon Cytokine Res. 2011. PMID: 21182352 Free PMC article. Review.
Cited by
-
MicroRNAs in diabetic nephropathy: functions, biomarkers, and therapeutic targets.Ann N Y Acad Sci. 2015 Sep;1353(1):72-88. doi: 10.1111/nyas.12758. Epub 2015 Apr 15. Ann N Y Acad Sci. 2015. PMID: 25877817 Free PMC article. Review.
-
Reduced adenosine-to-inosine miR-455-5p editing promotes melanoma growth and metastasis.Nat Cell Biol. 2015 Mar;17(3):311-21. doi: 10.1038/ncb3110. Epub 2015 Feb 16. Nat Cell Biol. 2015. PMID: 25686251 Free PMC article.
-
ADAR1p150 Forms a Complex with Dicer to Promote miRNA-222 Activity and Regulate PTEN Expression in CVB3-Induced Viral Myocarditis.Int J Mol Sci. 2019 Jan 18;20(2):407. doi: 10.3390/ijms20020407. Int J Mol Sci. 2019. PMID: 30669342 Free PMC article.
-
ADAR1-Dependent RNA Editing Promotes MET and iPSC Reprogramming by Alleviating ER Stress.Cell Stem Cell. 2020 Aug 6;27(2):300-314.e11. doi: 10.1016/j.stem.2020.04.016. Epub 2020 May 11. Cell Stem Cell. 2020. PMID: 32396862 Free PMC article.
-
Z-DNA and Z-RNA in human disease.Commun Biol. 2019 Jan 7;2:7. doi: 10.1038/s42003-018-0237-x. eCollection 2019. Commun Biol. 2019. PMID: 30729177 Free PMC article. Review.
References
-
- Cho DS, Yang W, Lee JT, Shiekhattar R, Murray JM, Nishikura K. Requirement of dimerization for RNA editing activity of adenosine deaminases acting on RNA. J. Biol. Chem. 2003;278:17093–17102. - PubMed
-
- Dlakic M. DUF283 domain of Dicer proteins has a double-stranded RNA-binding fold. Bioinformatics. 2006;22:2711–2714. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

