Acid shock of Listeria monocytogenes at low environmental temperatures induces prfA, epithelial cell invasion, and lethality towards Caenorhabditis elegans
- PMID: 23622257
- PMCID: PMC3648428
- DOI: 10.1186/1471-2164-14-285
Acid shock of Listeria monocytogenes at low environmental temperatures induces prfA, epithelial cell invasion, and lethality towards Caenorhabditis elegans
Abstract
Background: The saprophytic pathogen Listeria monocytogenes has to cope with a variety of acidic habitats during its life cycle. The impact of low-temperature coupled with pH decrease for global gene expression and subsequent virulence properties, however, has not been elucidated.
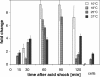
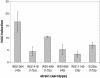
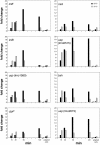
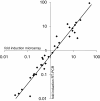
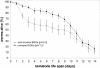
Results: qRT-PCR revealed for the first time a transient, acid triggered prfA induction of approximately 4-fold, 5.7-fold, 7-fold and 9.3-fold 60 to 90 min after acid shock of L. monocytogenes at 37°C, 25°C, 18°C, and 10°C, respectively. Comparable data were obtained for seven different L. monocytogenes strains, demonstrating that prfA induction under these conditions is a general response of L. monocytogenes. Transcriptome analysis revealed that the in vivo-relevant genes bsh, clpP, glpD, hfq, inlA, inlB, inlE, lisR, and lplA1 as well as many other genes with a putative role during infection are transiently induced upon acid shock conducted at 25°C and 37°C. Twenty-five genes repressed upon acid shock are known to be down regulated during intracellular growth or by virulence regulators. These data were confirmed by qRT-PCR of twelve differentially regulated genes and by the identification of acid shock-induced genes influenced by σB. To test if up regulation of virulence genes at temperatures below 37°C correlates with pathogenicity, the capacity of L. monocytogenes to invade epithelial cells after acid shock at 25°C was measured. A 12-fold increased number of intracellular bacteria was observed (acid shock, t = 60 min) that was reduced after adaptation to the level of the unshocked control. This increased invasiveness was shown to be in line with the induction of inlAB. Using a nematode infection assay, we demonstrated that Caenorhabditis elegans fed with acid-shocked L. monocytogenes exhibits a shorter time to death of 50% (TD50) of the worms (6.4 days) compared to infection with unshocked bacteria (TD50 = 10.2 days).
Conclusions: PrfA and other listerial virulence genes are induced by an inorganic acid in a temperature-dependent manner. The data presented here suggest that low pH serves as a trigger for listerial pathogenicity at environmental temperatures.
Figures

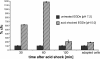
Similar articles
-
Growth temperature-dependent contributions of response regulators, σB, PrfA, and motility factors to Listeria monocytogenes invasion of Caco-2 cells.Foodborne Pathog Dis. 2010 Nov;7(11):1337-49. doi: 10.1089/fpd.2010.0563. Epub 2010 Aug 14. Foodborne Pathog Dis. 2010. PMID: 20707735 Free PMC article.
-
Contributions of Listeria monocytogenes sigmaB and PrfA to expression of virulence and stress response genes during extra- and intracellular growth.Microbiology (Reading). 2006 Jun;152(Pt 6):1827-1838. doi: 10.1099/mic.0.28758-0. Microbiology (Reading). 2006. PMID: 16735745
-
Evaluation of transcription levels of inlA, inlB, hly, bsh and prfA genes in Listeria monocytogenes strains using quantitative reverse-transcription PCR and ability of invasion into human CaCo-2 cells.FEMS Microbiol Lett. 2015 Mar;362(6):fnv018. doi: 10.1093/femsle/fnv018. Epub 2015 Feb 11. FEMS Microbiol Lett. 2015. PMID: 25673285
-
Cross Talk between SigB and PrfA in Listeria monocytogenes Facilitates Transitions between Extra- and Intracellular Environments.Microbiol Mol Biol Rev. 2019 Sep 4;83(4):e00034-19. doi: 10.1128/MMBR.00034-19. Print 2019 Nov 20. Microbiol Mol Biol Rev. 2019. PMID: 31484692 Free PMC article. Review.
-
Flick of a switch: regulatory mechanisms allowing Listeria monocytogenes to transition from a saprophyte to a killer.Microbiology (Reading). 2019 Aug;165(8):819-833. doi: 10.1099/mic.0.000808. Epub 2019 May 20. Microbiology (Reading). 2019. PMID: 31107205 Review.
Cited by
-
A comprehensive investigation of protein expression profiles in L. monocytogenes exposed to thermal abuse, mild acid, and salt stress conditions.Front Microbiol. 2023 Oct 9;14:1271787. doi: 10.3389/fmicb.2023.1271787. eCollection 2023. Front Microbiol. 2023. PMID: 37876777 Free PMC article.
-
Xenopus laevis as an infection model for human pathogenic bacteria.Infect Immun. 2025 Jun 10;93(6):e0012625. doi: 10.1128/iai.00126-25. Epub 2025 May 1. Infect Immun. 2025. PMID: 40310291 Free PMC article.
-
Ceviche-Natural Preservative: Possibility of Microbiota Survival and Effect on L. monocytogenes.Foods. 2022 Mar 18;11(6):860. doi: 10.3390/foods11060860. Foods. 2022. PMID: 35327282 Free PMC article.
-
Acid stress signals are integrated into the σB-dependent general stress response pathway via the stressosome in the food-borne pathogen Listeria monocytogenes.PLoS Pathog. 2022 Mar 11;18(3):e1010213. doi: 10.1371/journal.ppat.1010213. eCollection 2022 Mar. PLoS Pathog. 2022. PMID: 35275969 Free PMC article.
-
Effect of Temperatures Used in Food Storage on Duration of Heat Stress Induced Invasiveness of L. monocytogenes.Microorganisms. 2019 Oct 17;7(10):467. doi: 10.3390/microorganisms7100467. Microorganisms. 2019. PMID: 31627472 Free PMC article.
References
-
- Fenlon DR. In: Listeria, Listeriosis, and Food Safety. Volume 1. 2. Ryser ET, Marth EH, editor. New York: Marcel Dekker Inc; 1999. Listeria monocytogenes in the natural environment; pp. 21–38.
-
- Gahan CGM, Hill C. In: Microbial Stress Adaptation and Food Safety. Yousef AE, Juneja VK, editor. CRC Press; 2002. Relationship between stress adaptation and virulence in foodborne pathogenic bacteria.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

