Next generation biofuel engineering in prokaryotes
- PMID: 23623045
- PMCID: PMC4211605
- DOI: 10.1016/j.cbpa.2013.03.037
Next generation biofuel engineering in prokaryotes
Abstract
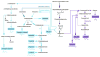
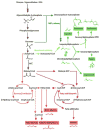
Next-generation biofuels must be compatible with current transportation infrastructure and be derived from environmentally sustainable resources that do not compete with food crops. Many bacterial species have unique properties advantageous to the production of such next-generation fuels. However, no single species possesses all characteristics necessary to make high quantities of fuels from plant waste or CO2. Species containing a subset of the desired characteristics are used as starting points for engineering organisms with all desired attributes. Metabolic engineering of model organisms has yielded high titer production of advanced fuels, including alcohols, isoprenoids, and fatty acid derivatives. Technical developments now allow engineering of native fuel producers, as well as lignocellulolytic and autotrophic bacteria, for the production of biofuels. Continued research on multiple fronts is required to engineer organisms for truly sustainable and economical biofuel production.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Conflict of interest statement
J.C.L. is a cofounder of Easel Biotechnologies, which has licensed biofuel technology from the University of California, Los Angeles.
Figures


References
-
- Tuck CO, Perez E, Horvath IT, Sheldon RA, Poliakoff M. Valorization of biomass: deriving more value from waste. Science. 2012;337:695–699. - PubMed
-
- Peralta-Yahya PP, Zhang F, del Cardayre SB, Keasling JD. Microbial engineering for the production of advanced biofuels. Nature. 2012;488:320–328. - PubMed
-
- Inokuma K, Liao JC, Okamoto M, Hanai T. Improvement of isopropanol production by metabolically engineered Escherichia coli using gas stripping. Journal of bioscience and bioengineering. 2010;110:696–701. - PubMed
-
- Bond-Watts BB, Bellerose RJ, Chang MC. Enzyme mechanism as a kinetic control element for designing synthetic biofuel pathways. Nature chemical biology. 2011;7:222–227. This paper describes assembly of a butanol pathway using enzymes from different sources selected based on their reaction mechanism to kinetically trap the product. 28% of theoretical yield from glucose is achieved. - PubMed
-
- Shen CR, Lan EI, Dekishima Y, Baez A, Cho KM, Liao JC. Driving forces enable high-titer anaerobic 1-butanol synthesis in Escherichia coli. Applied and environmental microbiology. 2011;77:2905–2915. The authors introduce an irreversible enzyme into the butanol pathway and create NADH and acetyl-CoA driving forces to achieve 70–88% of theoretical yield of butanol from glucose. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

