Malfunction of nuclease ERCC1-XPF results in diverse clinical manifestations and causes Cockayne syndrome, xeroderma pigmentosum, and Fanconi anemia
- PMID: 23623389
- PMCID: PMC3644632
- DOI: 10.1016/j.ajhg.2013.04.007
Malfunction of nuclease ERCC1-XPF results in diverse clinical manifestations and causes Cockayne syndrome, xeroderma pigmentosum, and Fanconi anemia
Abstract
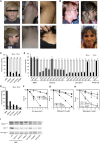
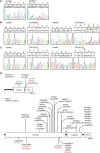
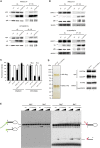
Cockayne syndrome (CS) is a genetic disorder characterized by developmental abnormalities and photodermatosis resulting from the lack of transcription-coupled nucleotide excision repair, which is responsible for the removal of photodamage from actively transcribed genes. To date, all identified causative mutations for CS have been in the two known CS-associated genes, ERCC8 (CSA) and ERCC6 (CSB). For the rare combined xeroderma pigmentosum (XP) and CS phenotype, all identified mutations are in three of the XP-associated genes, ERCC3 (XPB), ERCC2 (XPD), and ERCC5 (XPG). In a previous report, we identified several CS cases who did not have mutations in any of these genes. In this paper, we describe three CS individuals deficient in ERCC1 or ERCC4 (XPF). Remarkably, one of these individuals with XP complementation group F (XP-F) had clinical features of three different DNA-repair disorders--CS, XP, and Fanconi anemia (FA). Our results, together with those from Bogliolo et al., who describe XPF alterations resulting in FA alone, indicate a multifunctional role for XPF.
Copyright © 2013 The American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Kleijer W.J., Laugel V., Berneburg M., Nardo T., Fawcett H., Gratchev A., Jaspers N.G., Sarasin A., Stefanini M., Lehmann A.R. Incidence of DNA repair deficiency disorders in western Europe: Xeroderma pigmentosum, Cockayne syndrome and trichothiodystrophy. DNA Repair (Amst.) 2008;7:744–750. - PubMed
-
- Laugel V., Dalloz C., Tobias E.S., Tolmie J.L., Martin-Coignard D., Drouin-Garraud V., Valayannopoulos V., Sarasin A., Dollfus H. Cerebro-oculo-facio-skeletal syndrome: three additional cases with CSB mutations, new diagnostic criteria and an approach to investigation. J. Med. Genet. 2008;45:564–571. - PubMed
-
- Friedberg E.C., Walker G.C., Siede W., Wood R.D., Schultz R.A., Ellenberger T. ASM Press; Washington, DC: 2006. DNA Repair and Mutagenesis, Second Edition.
-
- Hanawalt P.C., Spivak G. Transcription-coupled DNA repair: two decades of progress and surprises. Nat. Rev. Mol. Cell Biol. 2008;9:958–970. - PubMed
-
- Laugel V., Dalloz C., Durand M., Sauvanaud F., Kristensen U., Vincent M.C., Pasquier L., Odent S., Cormier-Daire V., Gener B. Mutation update for the CSB/ERCC6 and CSA/ERCC8 genes involved in Cockayne syndrome. Hum. Mutat. 2010;31:113–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

