Effect of anchoring 4-anilidopiperidines to opioid peptides
- PMID: 23623418
- PMCID: PMC3942532
- DOI: 10.1016/j.bmcl.2013.03.065
Effect of anchoring 4-anilidopiperidines to opioid peptides
Abstract
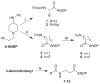
We report here the design, synthesis, and in vitro characterization of new opioid peptides featuring a 4-anilidopiperidine moiety. Despite the fact that the chemical structures of fentanyl surrogates have been found suboptimal per se for the opioid activity, the corresponding conjugates with opioid peptides displayed potent opioid activity. These studies shed an instructive light on the strategies and potential therapeutic values of anchoring the 4-anilidopiperidine scaffold to different classes of opioid peptides.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Understanding the structural requirements of 4-anilidopiperidine analogues for biological activities at mu and delta opioid receptors.Bioorg Med Chem Lett. 2007 Apr 15;17(8):2161-5. doi: 10.1016/j.bmcl.2007.01.114. Epub 2007 Feb 8. Bioorg Med Chem Lett. 2007. PMID: 17329100 Free PMC article.
-
Structural biology: How opioid drugs bind to receptors.Nature. 2012 May 16;485(7398):314-7. doi: 10.1038/485314a. Nature. 2012. PMID: 22596150 Free PMC article.
-
Design of a high affinity peptidomimetic opioid agonist from peptide pharmacophore models.Bioorg Med Chem Lett. 1998 Oct 6;8(19):2685-8. doi: 10.1016/s0960-894x(98)00472-7. Bioorg Med Chem Lett. 1998. PMID: 9873603
-
Cloning and characterization of multiple opioid receptors.NIDA Res Monogr. 1996;161:127-40. NIDA Res Monogr. 1996. PMID: 8784848 Review.
-
Multivalent peptide and peptidomimetic ligands for the treatment of pain without toxicities and addiction.Peptides. 2019 Jun;116:63-67. doi: 10.1016/j.peptides.2019.02.004. Epub 2019 Apr 20. Peptides. 2019. PMID: 31014958 Review.
Cited by
-
Fentanyl Family at the Mu-Opioid Receptor: Uniform Assessment of Binding and Computational Analysis.Molecules. 2019 Feb 19;24(4):740. doi: 10.3390/molecules24040740. Molecules. 2019. PMID: 30791394 Free PMC article.
-
A chemically contiguous hapten approach for a heroin-fentanyl vaccine.Beilstein J Org Chem. 2019 May 3;15:1020-1031. doi: 10.3762/bjoc.15.100. eCollection 2019. Beilstein J Org Chem. 2019. PMID: 31164940 Free PMC article.
-
Discovery of 5-substituted tetrahydronaphthalen-2yl-methyl with N-phenyl-N-(piperidin-4-yl)propionamide derivatives as potent opioid receptor ligands.Bioorg Med Chem. 2015 Sep 15;23(18):6185-94. doi: 10.1016/j.bmc.2015.07.071. Epub 2015 Aug 4. Bioorg Med Chem. 2015. PMID: 26299827 Free PMC article.
References
-
- Stanley TH. J Pain Symptom Manage. 1992;7:S3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information

