Antagonism between retinoic acid and fibroblast growth factor signaling during limb development
- PMID: 23623500
- PMCID: PMC3745640
- DOI: 10.1016/j.celrep.2013.03.036
Antagonism between retinoic acid and fibroblast growth factor signaling during limb development
Abstract
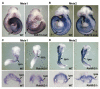
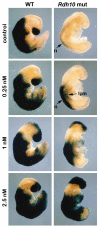
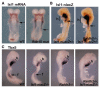
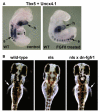
The vitamin A metabolite retinoic acid (RA) provides patterning information during vertebrate embryogenesis, but the mechanism through which RA influences limb development is unclear. During patterning of the limb proximodistal axis (upper limb to digits), avian studies suggest that a proximal RA signal generated in the trunk antagonizes a distal fibroblast growth factor (FGF) signal. However, mouse and zebrafish genetic studies suggest that loss of RA suppresses forelimb initiation. Here, using genetic and pharmacological approaches, we demonstrate that limb proximodistal patterning is not RA dependent, thus indicating that RA-FGF antagonism does not occur along the proximodistal axis of the limb. Instead, our studies show that RA-FGF antagonism acts prior to limb budding along the anteroposterior axis of the trunk lateral plate mesoderm to provide a patterning cue that guides formation of the forelimb field. These findings reconcile disparate ideas regarding RA-FGF antagonism and provide insight into how endogenous RA programs the early embryo.
Copyright © 2013 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



Comment in
-
Redefining the role of retinoic acid in limb development.Cell Rep. 2013 May 30;3(5):1337-8. doi: 10.1016/j.celrep.2013.05.010. Cell Rep. 2013. PMID: 23726021
Similar articles
-
Opposing RA and FGF signals control proximodistal vertebrate limb development through regulation of Meis genes.Development. 2000 Sep;127(18):3961-70. doi: 10.1242/dev.127.18.3961. Development. 2000. PMID: 10952894
-
Retinoic acid promotes limb induction through effects on body axis extension but is unnecessary for limb patterning.Curr Biol. 2009 Jun 23;19(12):1050-7. doi: 10.1016/j.cub.2009.04.059. Epub 2009 May 21. Curr Biol. 2009. PMID: 19464179 Free PMC article.
-
Embryonic retinoic acid synthesis is required for forelimb growth and anteroposterior patterning in the mouse.Development. 2002 Aug;129(15):3563-74. doi: 10.1242/dev.129.15.3563. Development. 2002. PMID: 12117807
-
Role of Retinoic Acid Signaling, FGF Signaling and Meis Genes in Control of Limb Development.Biomolecules. 2021 Jan 9;11(1):80. doi: 10.3390/biom11010080. Biomolecules. 2021. PMID: 33435477 Free PMC article. Review.
-
The Role of Retinoic Acid in Establishing the Early Limb Bud.Biomolecules. 2020 Feb 17;10(2):312. doi: 10.3390/biom10020312. Biomolecules. 2020. PMID: 32079177 Free PMC article. Review.
Cited by
-
The Cdx transcription factors and retinoic acid play parallel roles in antero-posterior position of the pectoral fin field during gastrulation.Mech Dev. 2020 Dec;164:103644. doi: 10.1016/j.mod.2020.103644. Epub 2020 Sep 8. Mech Dev. 2020. PMID: 32911082 Free PMC article.
-
RA Acts in a Coherent Feed-Forward Mechanism with Tbx5 to Control Limb Bud Induction and Initiation.Cell Rep. 2015 Aug 4;12(5):879-91. doi: 10.1016/j.celrep.2015.06.068. Epub 2015 Jul 23. Cell Rep. 2015. PMID: 26212321 Free PMC article.
-
A novel method to reconstruct epithelial tissue using high-purity keratinocyte lineage cells induced from human embryonic stem cells.Cell Cycle. 2019 Feb;18(3):264-273. doi: 10.1080/15384101.2018.1555118. Epub 2019 Jan 8. Cell Cycle. 2019. PMID: 30563408 Free PMC article.
-
Low retinoic acid levels mediate regionalization of the Sertoli valve in the terminal segment of mouse seminiferous tubules.Sci Rep. 2021 Jan 13;11(1):1110. doi: 10.1038/s41598-020-79987-4. Sci Rep. 2021. PMID: 33441739 Free PMC article.
-
Sall genes regulate hindlimb initiation in mouse embryos.Genetics. 2024 May 7;227(1):iyae029. doi: 10.1093/genetics/iyae029. Genetics. 2024. PMID: 38386912 Free PMC article.
References
-
- Agarwal P, Wylie JN, Galceran J, Arkhitko O, Li C, Deng C, Grosschedl R, Bruneau BG. Tbx5 is essential for forelimb bud initiation following patterning of the limb field in the mouse embryo. Development. 2003;130:623–633. - PubMed
-
- Begemann G, Schilling TF, Rauch GJ, Geisler R, Ingham PW. The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development. 2001;128:3081–3094. - PubMed
-
- Bruneau BG, Nemer G, Schmitt JP, Charron F, Robitaille L, Caron S, Conner DA, Gessler M, Nemer M, Seidman CE, Seidman JG. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell. 2001;106:709–721. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

