Modification of agonist binding moiety in hybrid derivative 5/7-{[2-(4-aryl-piperazin-1-yl)-ethyl]-propyl-amino}-5,6,7,8-tetrahydro-naphthalen-1-ol/-2-amino versions: impact on functional activity and selectivity for dopamine D2/D3 receptors
- PMID: 23623679
- PMCID: PMC3760392
- DOI: 10.1016/j.bmc.2013.03.059
Modification of agonist binding moiety in hybrid derivative 5/7-{[2-(4-aryl-piperazin-1-yl)-ethyl]-propyl-amino}-5,6,7,8-tetrahydro-naphthalen-1-ol/-2-amino versions: impact on functional activity and selectivity for dopamine D2/D3 receptors
Abstract
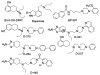
The goal of the present study was to explore, in our previously developed hybrid template, the effect of introduction of additional heterocyclic rings (mimicking catechol hydroxyl groups as bioisosteric replacement) on selectivity and affinity for the D3 versus D2 receptor. In addition, we wanted to explore the effect of derivatization of functional groups of the agonist binding moiety in compounds developed by us earlier from the hybrid template. Binding affinity (K(i)) of the new compounds was measured with tritiated spiperone as the radioligand and HEK-293 cells expressing either D2 or D3 receptors. Functional activity of selected compounds was assessed in the GTPγS binding assay. In the imidazole series, compound 10a exhibited the highest D3 affinity whereas the indole derivative 13 exhibited similar high D3 affinity. Functionalization of the amino group in agonist (+)-9d with different sulfonamides derivatives improved the D3 affinity significantly with (+)-14f exhibiting the highest affinity. However, functionalization of the hydroxyl and amino groups of 15 and (+)-9d, known agonist and partial agonist, to sulfonate ester and amide in general modulated the affinity. In both cases loss of agonist potency resulted from such derivatization.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures


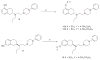
Similar articles
-
Synthesis and biological characterization of novel hybrid 7-[[2-(4-phenyl-piperazin-1-yl)-ethyl]-propyl-amino]-5,6,7,8-tetrahydro-naphthalen-2-ol and their heterocyclic bioisosteric analogues for dopamine D2 and D3 receptors.Bioorg Med Chem. 2004 Aug 15;12(16):4361-73. doi: 10.1016/j.bmc.2004.06.019. Bioorg Med Chem. 2004. PMID: 15265488
-
Investigation of various N-heterocyclic substituted piperazine versions of 5/7-{[2-(4-aryl-piperazin-1-yl)-ethyl]-propyl-amino}-5,6,7,8-tetrahydro-naphthalen-2-ol: effect on affinity and selectivity for dopamine D3 receptor.Bioorg Med Chem. 2009 Jun 1;17(11):3923-33. doi: 10.1016/j.bmc.2009.04.031. Epub 2009 Apr 19. Bioorg Med Chem. 2009. PMID: 19427222 Free PMC article.
-
Further structure-activity relationships study of hybrid 7-{[2-(4-phenylpiperazin-1-yl)ethyl]propylamino}-5,6,7,8-tetrahydronaphthalen-2-ol analogues: identification of a high-affinity D3-preferring agonist with potent in vivo activity with long duration of action.J Med Chem. 2008 Jan 10;51(1):101-17. doi: 10.1021/jm070860r. Epub 2007 Dec 12. J Med Chem. 2008. PMID: 18072730
-
Bioisosteric heterocyclic versions of 7-{[2-(4-phenyl-piperazin-1-yl)ethyl]propylamino}-5,6,7,8-tetrahydronaphthalen-2-ol: identification of highly potent and selective agonists for dopamine D3 receptor with potent in vivo activity.J Med Chem. 2008 May 22;51(10):3005-19. doi: 10.1021/jm701524h. Epub 2008 Apr 12. J Med Chem. 2008. PMID: 18410082
-
Revision of the classical dopamine D2 agonist pharmacophore based on an integrated medicinal chemistry, homology modelling and computational docking approach.Neurochem Res. 2014 Oct;39(10):1997-2007. doi: 10.1007/s11064-014-1314-2. Epub 2014 Jul 24. Neurochem Res. 2014. PMID: 25056287 Review.
Cited by
-
Design, Synthesis, and Biological Evaluation of Phenol Bioisosteric Analogues of 3-Hydroxymorphinan.Sci Rep. 2019 Feb 19;9(1):2247. doi: 10.1038/s41598-019-38911-1. Sci Rep. 2019. PMID: 30783196 Free PMC article.
-
Multicomponent Synthesis and Biological Evaluation of a Piperazine-Based Dopamine Receptor Ligand Library.ACS Med Chem Lett. 2015 Jun 23;6(8):882-7. doi: 10.1021/acsmedchemlett.5b00131. eCollection 2015 Aug 13. ACS Med Chem Lett. 2015. PMID: 26288260 Free PMC article.
-
Synthesis and Cytotoxic Evaluation of Pyrrole Hetarylazoles Containing Benzimidazole/Pyrazolone/1,3,4-Oxadiazole Motifs.J Heterocycl Chem. 2016 Nov;53(6):1871-1877. doi: 10.1002/jhet.2501. Epub 2015 Sep 18. J Heterocycl Chem. 2016. PMID: 27956751 Free PMC article.
-
Synthesis and structure-activity relationships for a new class of tetrahydronaphthalene amide inhibitors of Mycobacterium tuberculosis.Eur J Med Chem. 2022 Feb 5;229:114059. doi: 10.1016/j.ejmech.2021.114059. Epub 2021 Dec 21. Eur J Med Chem. 2022. PMID: 34963068 Free PMC article.
References
-
- Emilien G, Maloteaux JM, Geurts M, Hoogenberg K, Cragg S. Pharmacol Ther. 1999;84:133. - PubMed
-
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Physiol Rev. 1998;78:189. - PubMed
-
- Kebabian JW, Calne DB. Nature. 1979;277:93. - PubMed
-
- Giros B, Martres MP, Sokoloff P, Schwartz JC. C R Biol. 1990;311:501. - PubMed
-
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC. Nature. 1990;347:146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

